Key words
Antibiotic susceptibility, Brucella spp, Brucellosis treatment, E-Test
Introduction
Human brucellosis is most frequently caused by B. melitensis. In addition to this, other species has also been diagnosed in human beings. Brucellosis is a widespread disease of various animal species, and causes a common zoonotic infection of humans in many countries in the world and especially in the Mediterranean areas [1-3].
The genus Brucella is divided into six classical species. Four of six Brucella species (B. abortus, B. melitensis, B. ovis, B. canis, B. suis, and B. neotomae) may cause human infection. B. melitensis is the most common cause of infection, followed by B. abortus and B. suis. B. canis infections are rarely described in humans [4,5].
Brucella are intracellular bacterial pathogens that infect host macrophage cells. In consequence, specialized agents that are able to penetrate the macrophages and function within their cytoplasm are required for the treatment of brucellosis [6]. According to World Health Organization (WHO) guidelines, the recommended combination of two antibiotics can be used for the treatment of brucellosis. WHO recommended regimen is doxycycline (DOX) in combination with rifampicin (RIF) for 6 weeks. The combination of DOX and streptomycin (STR) is also effective. Although Brucella isolates are generally considered susceptible to recommended antibiotics, sporadic cases of antibiotic resistance and disease relapse have been reported [7]. Drug resistance is a particularly important issue as most people infected with brucellosis live in low socioeconomic areas of developing countries, where tuberculosis is also an endemic health problem. Thus, there are concerns over the potential increase in resistance to tuberculosis drugs due to their prolonged use in treating brucellosis [8].
Antimicrobial susceptibility tests for Brucella haven’t been standardized yet, and routine susceptibility tests can’t be performed in microbiology laboratories. The break point values haven’t described clearly yet [9,10].
The aim of this study is to identify Brucella strains isolated from various clinical specimens and determine their in-vitro antimicrobial susceptibilities to DOX, STR, RIF, ciprofloxacin (CIP), tigecycline (TGC), gentamycin (GEN), trimethoprimsulfamethoxsazole (SXT), erythromycin (EM), ampicillin (AMP) and amoxicillin/clavulonic acid (AMC) using E-test method.
Materials and Methods
A total of 50 Brucella strains isolated from various clinical specimens at the Central Laboratory of Cukurova University Balcali Hospital between January 2010 and October 2012 were included in this study. Brucella strains were isolated from blood (n=45), CSF (n=2), nephrostomy (n=1), abscess (n=1) and synovial fluid (n=1). Blood cultures were incubated in vials of the BACTEC 9240 system (Becton Dickinson, Rutherford, NJ) at 37°C for 7 days. Positive signals were recorded and the samples were inoculated into 5% sheep blood agar (COS; bioMerieux) twice, and incubated with and without 5% CO2 for 48-72 h at 37oC. After incubation, Gram-negative coccobacilli which were oxidase and catalase positive were identified by the Vitek 2 automated system. The strains identified as B. melitensis were stored in microbank tubes at -20°C until susceptibility testing. On the other hand, the isolates were tested for agglutination with monospecific anti- Brucella serum (Remel Inc., Lenexa, Kans.). All Brucella isolates were identified as B. melitensis.
Testing antimicrobial susceptibility
Antimicrobial susceptibility testing of the Brucella isolates to ten antibiotics- DOX, STR, RIF, CIP, TGC, GEN, SXT, EM, AMP and AMC- was performed by E-test method. E-test strips were stored at -20°C until use. An inoculum equal to a 0.5 McFarland turbidity standard was prepared from each Brucella isolate, and bacterial suspension was inoculated onto Mueller-Hinton agar plates supplemented with 5% sheep blood. The E-test strips were applied to the inoculated culture plates separately as recommended by the manufacturer, and the plates were incubated at 37°C for 48 h under aerobic conditions. Determination of the MIC was performed in accordance with the recommended reference values of the Clinical Laboratory Standards Institute’s (CLSI) guidelines to DOX, STR, GEN, SXT for Brucella spp and RIF, CIP, AMP, AMC for slow-growing bacteria (Haemophilus spp.). The MIC50 and MIC90 values, which indicate that the relevant concentration inhibits the growth of 50% or 90% of the bacteria, respectively, of the tested population were determined. All tests were performed by biosafety level 3 cabinets. Such testing carries the risk of contagious among laboratory personnel.
Reference strains
The reference strains Escherichia coli ATCC 25922 and Staphylococcus aures ATCC 29213 were used as quality controls.
Results
Thirty-three (66%) of the 50 strains were obtained from male patients, and seventeen (34%) were obtained from female patients. Samples had been sent from infectious disease (n=21), pediatrics (n=12), gastroenterology (n=2), brain surgery (n=2), orthopedics (n=2), hematology (n=2), internal medicine (n=1), general surgery (n=1), cardiology (n=1), rheumatology (n=1), otorhinolaryngology (n=1), urology (n=1), physical therapy and rehabilitation (n=1), burn unit (n=1) and cardiovascular surgery (n=1) departments of Cukurova University Balcali Hospital.
Using the BACTEC 9240 automated blood culture system, we detected all cultures positive for B. melitensis within six days of incubation. Moreover, 39 of 45 (87%) blood cultures were detected positive within first three days of incubation (Figure 1).
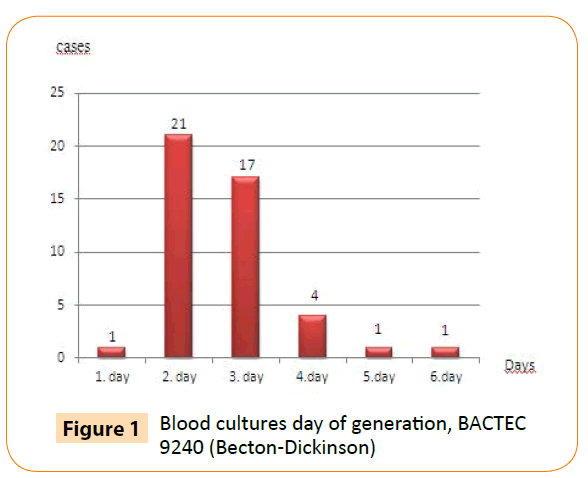
Figure 1: Blood cultures day of generation, BACTEC 9240 (Becton-Dickinson)
According to antibiotic susceptibility testing, 38 of the 50 B. melitensis strains were susceptible to RIF, 11 strains were intermediate-resistant and one strain was resistant to RIF. All strains were found to be suspectible to other antibiotics (Table 1). EM and TGC were included in the present study for research purposes only. Those agents aren’t defined by CLSI standards.
| Antimicrobial |
Susceptible |
Intermediate susceptible |
Resistant |
| agents |
n |
% |
n |
% |
n |
% |
| DOX |
50 |
100 |
- |
- |
- |
- |
| STR |
50 |
100 |
- |
- |
- |
- |
| RIF |
38 |
76 |
11 |
22 |
1 |
2 |
| CIP |
50 |
100 |
- |
- |
- |
- |
| GEN |
50 |
100 |
- |
- |
- |
- |
| SXT |
50 |
100 |
- |
- |
- |
- |
| AMP |
50 |
100 |
- |
- |
- |
- |
| AMC |
50 |
100 |
- |
- |
- |
- |
Table 1: Antibiotic susceptibilities of B. melitensis isolates.
According to MIC50 and MIC90 values, SXT (MIC50; 0.023 ug/ml and MIC90; 0.064 ug/ml) was the most effective antibiotic against B. melitensis strains. After SXT, the most effective antibiotics were GEN (MIC50; 0.047 ug/ml, MIC90; 0.094 ug/ml) and DOX (MIC50; 0.064 ug/ml, MIC90; 0.094 ug/ml), respectively. The highest MIC50 and MIC90 values had EM and RIF respectively. EM is ineffective in-vivo for brucellosis treatment (Table 2).
| Antimicrobial agents |
MIC ranges |
MIC50 |
MIC90 |
CLSI Breakpoints for Brucella (μg/ml) |
| (ug/ml) |
(ug/ml) |
(ug/ml) |
S |
I |
R |
| DOX |
0.047-0.19 |
0.064 |
0.094 |
≤1 |
- |
- |
| STR |
0.25-0.5 |
0.25 |
0.38 |
≤8 |
- |
- |
| RIF |
0.38-4 |
1 |
1.5 |
≤ 1* |
2* |
≥ 4* |
| CIP |
0.094-0.19 |
0.125 |
0.19 |
≤ 1* |
- |
- |
| GEN |
0.032-0.125 |
0.047 |
0.094 |
≤4 |
- |
- |
| SXT |
0.008-0.38 |
0.023 |
0.064 |
≤ 2/38 |
- |
- |
| EM** |
0.25-2 |
1.5 |
2 |
|
|
|
| AMP |
0.064-0.5 |
0.125 |
0.38 |
≤1* |
2* |
≥ 4* |
| AMC |
0.032-0.094 |
0.064 |
0.094 |
≤ 4/2* |
- |
≥8/4* |
| TGC** |
0.019-0.125 |
0.094 |
0.125 |
|
|
|
*CLSI breakpoints for slow-growing bacteria (Haemophilus spp.).
**Not defined by CLSI standards
Table 2: MIC ranges, MIC50 and MIC90 values of ten antibiotics against B. melitensis isolates.
Discussion
Brucellosis is still an important health problem in developing countries and leads to serious economic losses. The disease causes abortion and sterility in animals and septicemia those progresses to chronic localized infections in various organs of humans. Although brucellosis has been eradicated from animals in some developed countries, 500,000 new cases are reported yearly throughout the world, and it is still a widespread zoonotic disease in Turkey [9,11].
Brucella spp are highly infectious pathogens. Routine in-vitro antimicrobial susceptibility testing of Brucella spp. is not generally recommended [12-14].
Such testing carries the risk of contagiousness among laboratory personnel and requires level 3 biosafety precautions [6,12,14]. Additionally, there is no standardized method for susceptibility testing recommended by CLSI for these microorganisms [6].
In-vitro efficacy of antibiotics against Brucella spp. has usually been based on the determination of MIC values by micro broth dilution, agar dilution, and E-test methods. The disc diffusion method has not been recommended [14]. Most studies from Turkey utilized the E-test method and usually the results are similar [6,15]. E-test is a reliable, reproducible, and practical as well as less labor-intensive and time-consuming than other methods for antimicrobial susceptibility testing of Brucella strains [10,16]. Brucella agar, Muller-Hinton agar, and Muller- Hinton broth supplemented with 1% Polyvitex, or a combination of 1% Polyvitex and 1% haemoglobin, and Muller-Hinton agar supplemented with 5% sheep blood agar are the media used for antibiotic susceptibility testing of Brucella [10,14].
To achieve effective treatment, antimicrobials that can penetrate the cell at high concentrations should be chosen, and the duration of the therapy should be set properly [9]. DOX; has become the most commonly prescribed tetracycline derivative in the treatment of Brucella infections because of its superior pharmacokinetic features [17]. We found that DOX was not as effective as SXT. DOX had the highier MIC values than SXT.
SXT is an agent recommended for the treatment of brucellosis. It is used in combination with RIF in pregnant women and children under 8 years old, who cannot use tetracycline. A combination of SXT, DOX and RIF is successfully used in the treatment of Brucella endocarditis, which is the brucellosis complication with the highest mortality rate [18]. A study from Egypt by Maksoud et al., reported that SXT is an effective antibiotic with low MIC levels (MIC50; 0.047 μg/ml and MIC90; 0.19 μg/ml) [7]. Our study showed that SXT had the lowest MIC50 and MIC90 values. SXT was found to be the most effective antibiotic [19]. As well as our study, Aliskan et al. reported SXT as the most effective antimicrobial agent with the lowest MIC50 and MIC90 values [20].
RIF is a potent antibiotic in the treatment of Brucella infections, and it is widely accepted in the best first-line therapy [21]. Depending on its concentration, this antibiotic can have bacteriostatic or bactericidal effects. RIF can have bactericidal activity against slow and irregularly growing Mycobacterium tuberculosis organisms and it also plays a significant role in the treatment of Brucella species [22]. Several studies showed that RIF had excellent anti-Brucella activity, which accounts for its good intracellular penetration and clear synergism in combination with therapies which are recommended by the WHO antibiotics for the treatment of brucellosis [23].
RIF demonstrated the highest MIC values (0.38-4 μg/ml), with 22% of the isolates showing reduced susceptibility and 2% probable resistance, according to CLSI criteria for slow-growing bacteria. To our knowledge, this is the first report of resistance to RIF among B. melitensis isolates from Adana. The emergence of strains of intermediate sensitivity and resistance to RIF is likely due to the frequent usage of RIF as an antitubercular agent in long-term, multi-drug tuberculosis therapy in Turkey, which is accepted as an endemic region for tuberculosis. Some previous studies show that RIF has been intermediate-sensitive. In Adana, Aliskan et al. found that, from 65 isolates containing B. melitensis strains isolated from bone marrow and blood, 8 showed intermediate sensitivity to RIF. In Van Parlak et al., found that, from a total of 75 strains, 34 were found to have intermediate sensitivity to RIF [19]. Reduced susceptibility in 158 isolates (45%) was demonstrated by Maksoud et al in Egypt [24]. In another study conducted in Peru, only one Brucella isolate demonstrated reduced susceptibility to RIF [18].
Since decreasing suspectibility to RIF has been reported in many parts of the world, we suggest periodic assessment of susceptibility of strains to those antibiotics used most frequently in treatment, for an early detection of any drug resistance, especially in areas of endemicity [1].
Aminoglycosides penetrate human cells rather poorly, but have shown some intracellular activity after prolonged incubation [24]. In the present study, all Brucella isolates were susceptible to STR and GEN in agreement with previous studies from various countries [6,7,24,25]. In our study, MIC50 ve MIC90 (0.25 μg/ ml and 0.38 μg/ml) values of STR is relatively higher than GEN (0.047 μg/ml and 0.094 μg/ml). STR and GEN have been used clinically for the treatment of human brucellosis in tetracycline combinations.
Several studies focused on quinolones activity against Brucella, because these agents appeared as an attractive alternative drug choice for human brucellosis treatment [6]. Although fluoroquinolones had shown a high bactericidal activity against Brucella in-vitro, the in-vivo effectiveness of these antibiotics remains controversial [4,6]. MIC50 and MIC90 values were evaluated together and CIP was found to be one of the active agents by Köse et al. [3]. Our study revealed compatible results, suggesting that in-vitro CIP was as effective against B. melitensis strains.
TGC is a broad-spectrum glycylcycline antimicrobial agent and has been shown to be effective in-vitro against aerobic and anaerobic Gram-positive and Gram-negative microorganisms. Its activity against Brucella spp. has been investigated in several studies. As resistance breakpoints are not available for this agent in Brucella spp., in-vitro efficacies can be compared using MIC50 and MIC90 values. Baysan et al. reported 0.064 mg/l and 0.094 mg/l and Altun et al. reported 0.047 μg/ml and 0.094 μg/ml respectively MIC50 and MIC90 values for TGC [6,17]. Dizbay et al. reported TGC was more effective than RIF, SXT, STR, and DOX [13,17].
MIC50 and MIC90 values of TGC were 0.094 ug/ml and 0.125 ug/ ml respectively for our isolates. We found that TGC was more effective than STR, CIP and RIF but was not as effective as SXT, GEN and DOX. There are conflicting data about the MIC of TGC against Brucella in Turkey. Some in-vitro studies are needed to determine the efficacy of TGC in the treatment of brucellosis.
The role of macrolides in brucellosis treatment also remains controversial [6]. MIC values of EM ranged from 0.25-2 ug/ ml, indicating reduced activity. EM, AMP, and AMC acid were included in the study for research purposes only, as those agents are ineffective in-vivo for brucellosis treatment. Subsequently, the low MIC values of AMP (MIC50; 0.125 ug/ml, MIC90; 0.38 ug/ ml) and AMC (MIC50; 0.064 ug/ml, MIC90; 0.094 ug/ml) found in our isolates do not correspond to any therapeutic purpose.
Conclusion
Brucellosis remains a major public health problem in countries with low socioeconomical status. The necessity to keep RIF for tuberculosis treatment and the requirement of alternative drug therapy for specialized cases entails the research for other antibiotic usage. Our findings should alert us to the potential emergence of RIF’s resistance of Brucella in the region.
Antibiotic susceptibility patterns of Brucella spp. can differ from one geography to another. The establishment of a simple and reliable method for Brucella susceptibility testing would be useful for an early detection of any drug resistance that may be developed. Therefore, we suggest, regional periodic assessment of susceptibility of strains to antimicrobials.
Competing Interests
There are no competing interests
Funding
This work was supported by a grant from Scientific Research Projects Coordination Unit, Cukurova University (TF2010D9).
3807
References
- Marianelli, C., Graziani, C., Santangelo, C., Xibilia, MT., Imbriani, A., et al. Molecular epidemiological and antibiotic susceptibility characterization of Brucella isolates from humans in Sicily, Italy. J ClinMicrobiol 2007; 45: 2923-2928.
- Whatmore, AM., Perrett, LL., MacMillan, AP. Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol 2007; 7: 34.
- Köse, S., Kiliç, S., Ozbel, Y. Identification of Brucella species isolated from proven brucellosis patients in Izmir, Turkey. J Basic Microbiol2005; 45: 323-327.
- Al Dahouk, S., Nöckler, K., Hensel, A., Tomaso, H., Scholz, HC., et al. Human brucellosis in a nonendemiccountry: a report from Germany, 2002 and 2003. Eur J ClinMicrobiol Infect Dis 2005; 24: 450-456.
- Chen, Y., Zhen, Q., Wang, Y., Xu, J., Sun, Y., et al. Development of an extended multilocus sequence typing for genotyping of Brucellaisolates. Microbiol Methods 2011; 86: 252-254.
- Turkmani, A., Ioannidis, A., Christidou, A., Psaroulaki, A., Loukaides, F., et al. In vitro susceptibilities of Brucellamelitensis isolates to eleven antibiotics. Ann ClinMicrobiolAntimicrob 2006; 5: 24.
- Maksoud, MA., House, B., Wasfy, M., Rahman, BA., Pimentel, G., et al. In vitro antibiotic susceptibility testing of Brucella isolates from Egypt between 1999 and 2007 and evidence of probable rifampin resistance. Annals of Clinical Microbiology and Antimicrobials 2012; 11: 24.
- Yousefi-Nooraie, R., Mortaz-Hejri, S., Mehrani, M., Sadeghipour, P. Antibiotics for treating human brucellosis. Cochrane Database SystRev 2012; 10: CD007179.
- Orhan, G., Bayram, A., Zer, Y., Balci, I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucellamelitensis. J ClinMicrobiol 2005; 43: 140-143.
- SengÃz, G., YaÅŸar, KK.,Kutlu, SB., Durdu, YB., Ozdemir, R., et al. E-test susceptibility results of brucella strains for streptomycin, rifampicin, ciprofloxacin and tetracycline. MikrobiyolBul 2006; 40: 265-268.
- Celik, I., Akbulut, HH. Lymphocyte subpopulations in patients with acute brucellosis. Turk J Med Sci. 2005; 35: 237-41.
- Baykam, N., Esener, H., Ergönül, O., Eren S., Celikbas, AK., et al. In vitro antimicrobial susceptibility of Brucella species. Int J AntimicrobAgents 2004; 23: 405-407.
- Ayaslioglu, E., Kilic, S., Aydin, K., Kilic, D., Kaygusuz, S., et al. Antimicrobial susceptibility of Brucellamelitensis isolates from blood samples. Turk J Med Sci. 2008; 38: 257-262.
- Gür, D., Kocagöz, S., Akova, M., Unal, S. Comparison of E test tomicrodilution for determining in vitro activities of antibiotics against Brucellamelitensis. Antimicrob Agents Chemother 1999; 43: 2337.
- Yamazhan, T., Aydemir, S., Tünger, A., Serter, D., Gökengin, D. In vitro activities of various antimicrobials against Brucellamelitensis strains in the Aegean region in Turkey. Med PrincPract 2005; 14: 413-416.
- Bayram, Y., Korkoca, H., Aypak, C.,Parlak, M., Cikman, A., et al. Antimicrobial susceptibilities of Brucella isolates from various clinical specimens. Int J Med Sci 2011; 8: 198-202.
- Maves, RC., Castillo, R., Guillen, A., Espinosa, B., Meza R., et al. Antimicrobial susceptibility of Brucellamelitensis isolates in Peru. Antimicrob Agents Chemother 2011; 55: 1279-1281.
- Parlak, M., GÃÃÃoÄŸlu H, Bayram, Y., Aakman, A., Aypak, C., et al. Identification and determination of antibiotic susceptibilities of Brucella strains isolated from patients in van, Turkey by conventional and molecular methods. Int J Med Sci 2013; 10: 1406-1411.
- AliÅŸkan, H., TurunÃ, T., DemiroÄŸlu, YZ.,ColakoÄŸlu, S., Arslan, H. Short communication: Investigation of in vitro antibiotic susceptibility of Brucellamelitensis. MikrobiyolBul 2008; 42: 125-129.
- Marianelli, C., Ciuchini, F., Tarantino, M., Pasquali, P., Adone, R. Genetic bases of the rifampin resistance phenotype in Brucella spp. J ClinMicrobiol 2004; 42: 5439-5443.
- Sandalakis, V., Psaroulaki, A., De Bock, PJ.,Christidou, A., Gevaert, K., et al. Investigation of rifampicin resistance mechanisms in Brucellaabortus using MS-driven comparative proteomics. J Proteome Res 2012; 11: 2374-2385.
- Sayan, M., Kılıc, S., Uyanık, MH. Epidemiological survey of rifampicin resistance in clinic isolates of Brucellamelitensis obtained from all regions of Turkey. J Infect Chemother 2012; 18: 41-46.
- Dimitrov, Ts.,Panigrahi, D., Emara, M., Awni, F., Passadilla, R. Seroepidemiological and microbiological study of brucellosis in Kuwait. Med PrincPract 2004; 13: 215-219.
- Hashim, R., Ahmad, N., Zahidi, J., Tay, BY., Noor, AM., et al.Identification and In Vitro Antimicrobial Susceptibility of BrucellaSpecies Isolated from Human Brucellosis. Hindawi International Journal of Microbiology 2014; 5: 596245.






