Key words
|
| |
| Acridone, Cytotoxic, Antiviral, Antimicribial and patent |
| |
INTRODUCTION:
|
| |
| The journey of acridones as pharmaceuticals was started by Paul Ehrlich in late nineteenth century. Acridone is a tricyclic ring having nitrogen at 10th position and keto group at 9th position. Acridones, which belong to an important group of heterocyclic compounds due to their exceptional diverse activity attracted the scientific community and have been emerging as a new class of bioactive molecules. The ease of synthesis, attractive colouration and crytallinity of acridone compounds have attracted the attention of the chemists.1 Diversely substituted acridone and their derivatives embedded with variety of functional groups are important biological agents and a significant amount of research activity has been directed towards this class and they are used as antineoplastic, antimalarial, antibacterial, antifungal antiviral, anti-leishmanial and anti-inflammatory agents. The planar structure of these acridones facilitates to act on nucleotides by intercalating DNA and RNA strands, there by emerging as potent anticancer agents. They posses sufficient Hydrophilic Lipophilic Balance to traverse biological membranes to reach nucleus for exerting their action. Since they act on the nucleotides; they are also tried for antiviral, anti-AIDS and anti-fungal properties.2 A classical synthesis of these compounds involves the Ullmann condensation of aromatic halo acids and aromatic amines which produces anthranilic acid derivatives. These anthranilic acid derivatives on cyclization produces acridone moiety. In this method, cyclizing agents like conc. sulfuric acid, polyphosphoric acid phosphoryl chloride are used. In recent years, a significant portion of research in heterocyclic chemistry has been devoted to acridone containing different aryl groups as substituents, as evident from the literature. The preceding section of thereview is focusing on the recent development on acridones along with their biological properties. The last section of thereview dealt on various patents filed on acridones. |
| |
 |
| |
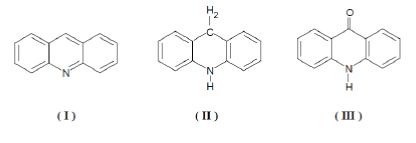
| Acridones are the oxidized products of acridines. Acridines have provided an important series of chemotherapeutic drugs, which range in complexity from the simple mono- and di- aminoacridines to the morecomplex acridines, which have proved to be specific for malaria and lambliasis (eg.Quinacrine and Acranil). Acridines are obtainable at three principle lavels of oxidation, of which thecommonest is the acridinelavel (I), Which passes on reduction to the acridanlavel (II) and on oxidation to the acridonelavel (III). Substances at one level of oxidation may rapidly be converted to any other, a fact that increases the number of synthetic routes available for any desired compound. |
| |
|
Nomenclature and Numbering
|
| |
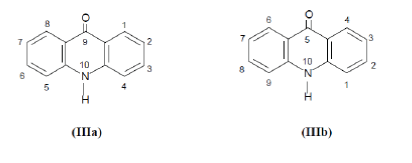
| Due to acrid smell and irritating action of acridone on skin and mucousmembrane, this substance was called ‘acridin’ (acris = short or pungent). Apart from the addition of a terminal ‘e’ and the replacement of ‘c’ by ‘k’ in a few older papers, these names have not subsequently been changed. The oxidized product of acridine wasnamed as ‘acridone’. In the beginning, various ways of numbering the acridone nucleus have beenadopted in different countries. In 1893, Graebe suggested a numbering system (IIIa) based on the then accepted numbering system used for anthracene, xanthene, etc.3 The system was generally accepted at the time. In 1900, M.M. Richter4 usedanother system (IIIb) in his ‘Lexikon der kohlenstoffverbindungen’. In 1937, chemical abstracts changed to (IIIa). |
| |
 |
| |
CHEMISTRY
|
| |
| The molecule is planer with no atoms deviating by more than 0.02 Å from the molecular plane defined by non-H ring atoms and the oxygen atoms, all torsion angle lies with in +1.5 to -1.5 of 0 to 180 degree. The molecules adopt a Harringbone Packing Arrangement very similar that found inanthraquinone&quinacridone. Hydrogen bonding is maximized in such structures.5 |
| |
| Synonyms of 9(10H)-acridone are: 9(10H)- Acridinone; Acridine-9-one; NIST 578-95-0;9- Acridanone; Acridine-9, 10-dihydro-9-oxo; 9, 10- dihydro-9-oxoacridine; 9-azanthracene-10-ones. |
| |
 |
| |
|
Physical Properties:
|
| |
| Acridone is a pure yellow solid, which melts at 354oC. It is almost insoluble in benzene, chloroform, ether, petroleum ether, water and ethanol. It can be recrystallized from acetic acid, amyl alcohol or mcresol and aniline acetate. Acridone dissolves in alcoholic potassium hydroxide to give a yellow brown solution of itspotassium salt, which is completely decomposed by water. The pka values for acridone are not known; potentiometric titration in extremely dilute aqueous alcoholic solution gave no inflection between pH 2 and 12.6 The high melting point and low solubility of acridone in many solvents indicates that it is a highly associatedsubstance. N-substituted acridones melt at much lower temperatures than the parent compound, suggesting that the N10-hydrogen atom is involved in the association. Substituents in the 4th and 5th positions also lower the melting points, perhaps reducing intermolecular association by a steric effect. These observations areconsistent with the suggestion of Hunter7 that acridones exhibit ‘mesohydric tautomerism’ or intermolecular hydrogen bonding. |
| |
|
Chemical Properties:-
|
| |
| Acridones yield acridines by zinc dust reduction and the reaction may beviolent if carried out on a large scale, with potassium cyanide it gave 9,10- dihydroacridine.8,9 Usually, the acridone is reduced to the correspondingacridan by an amalgum, which is then oxidized to the acridin. Acridone is fairly difficult to reduce nitroacridones. For instance, give only the corresponding amino-acridones with tin and hydrochloric acid or with hydrogen over Raney nickel. On oxidation with chromic acid acridone gave 10,10′-biacridonyl and 9(10)-acridine thione was formed on heating with sulfur and phosphorous. Acridone gave 9-phenylacridine with phenyl lithium and a mixture of 9-methylacridine and 9,9- dimethylacridan with methy lmagnesium iodide.10,11 The partial hydrolysis of 9-chloroacridine with 10% aqueous hydrochloricacid gave a bright red crystalline addition compound of one molecule of acridone to one of 9-chloroacridine hydrochloride. Similar addition compounds are formedbetween 2- methoxyacridone or its 10-methyl derivative and 2- methoxyacridine hydrochloride. 9-Phenyl aminoacridine or acridone anil was prepared from 9- aminoacridine and aniline. It has not been made from acridone and aniline, but gave these compounds on acid hydrolysis. Acridones substituted at position 2 with groups capable of donating a hydrogen atom to the ‘carbonyl’ show considerable intramolecular hydrogen bonding, which is intensified by Nalkylation. Substituents atposition 4 reduce intermolecular interaction by a steric effect and when the substituentcan accept a proton intramolecular hydrogen bonding, destroyed by N-alkylation, takes place. Inspite of the carbonyl group, neither oxime nor phenyl hydrozone isformed. |
| |
BIOLOGICAL ACTIVITIES OF ACRIDONES:-
|
| |
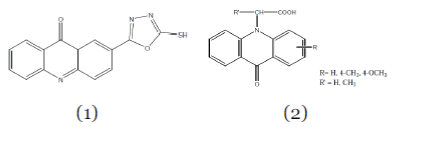
| 1. Antimicrobial and Antifungal Activity:- Salimon et al; (2010); synthesized 1, 3, 4 oxadiazole (Compound1) derivatives of acridone and screened them for antifungal and antibacterial activity. The results revealed that all thesynthesized compounds have a significant biological activity against the tested microorganisms.12 1, 3, 4 oxadiazole derivatives of acridone showed the significant antimicrobial activity against the microorganisms. |
| |
 |
| |
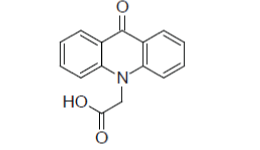
| Giridhar et al; (2010); synthesized the 9(10H) acridone derivatives (Compound 2). These compounds were evaluated for their antibacterial activity against Staphylococcus aureus, Bacillus subtilis and Escherichia coli. Among these compounds, 9-acridone-N-acetic acid, 9-acridone-N- 2-propionic acid, 2-methoxy-9-acridone-N-acetic acid showed good antibacterial activity.13 |
| |
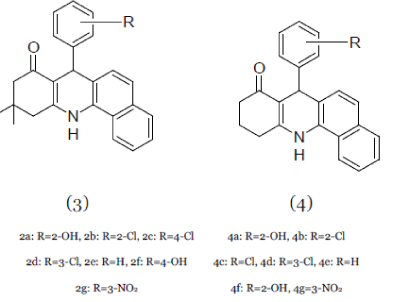
| Nadaraj et al; (2009); synthesized some new substituted tetrahydroacridin-8-ones and evaluated them for in-vitro anti-microbial activities. The compound 2a-g (3) and 4a-g (4) showed good inhibitory effect against the microorganisms such as Escherichia coli, Klebsiella aerogenes, Salmonella typhimurium, Bacillus cereus.14 |
| |
 |
| |
| Singh et al; (2009); synthesized some novel acridone derivatives and evaluated using Candida albicans as antifungal agent and investigate them for their effect on influx or efflux of Rhodamine 6G (R6G) in CA14 cells. Results indicate that the compound 5 inhibit the growth of CA14 cells and increase the influx/efflux of R6G in Ca14 cells.15 |
| |
 |
| |
|
2. Anti-Viral activity:-
|
| |
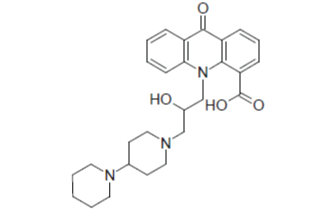
| Various acridone derivatives possess excellent antiviral activity. Acridone-10-yl acetic acid (Neovir) (6) is found to be effective as antiviral drug. |
| |
 |
| |
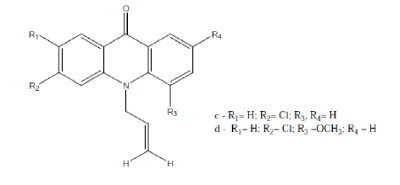
| Sepulveda et al; synthesized a series of Nsubstituted acridone (Compound 7) and evaluated against two haemorrhagic fever viruses. Among the tested compounds, two N-allyl acridones c and d elicited a potent and selective antiviral activity.16 |
| |
 |
| |
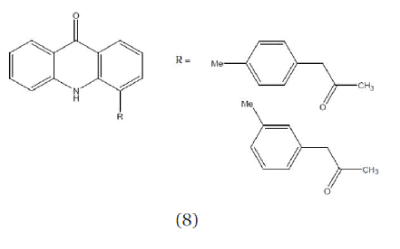
| Drogon et al; (2008); reported new acridone 4- carboxylic acid derivatives (Compound 8) and tested them as potential inhibitors of Hepatitis C virus infection. Two of the compounds N-pyridine-4-yl) – amide and N-(pyridine-2-yl)-amide of acridone-4- carboxylic acid are efficient RNA replication inhibitors with selectively indexes of 19.4 and 40.5 respectively.17 |
| |
 |
| |
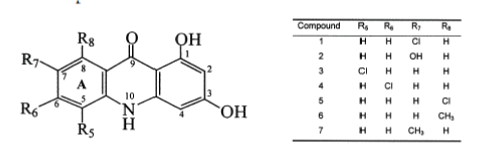
| Akanitapichat P. et al; (2000); reported 1,3- Dihydroxyacridone derivatives as inhibitors of herpes virus replication. Among all, 5-chloro,1,3- dihydroxyacridone (compound 3) was identified as a novel antiviral lead molecule. It is the inhibitor of HSV replication in cell culture.18 |
| |
 |
| |
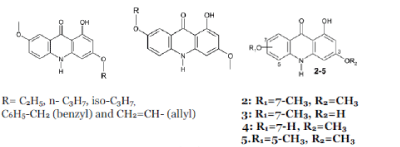
| Bastow K. F. et al; (2003); synthesized 3,7- dialkoxylated, 1-hydroxyacridone derivatives and checked inhibition of Cell culture replication of herpes simplex virus and, or human cytomegalovirus.19 |
| |
 |
| |
|
3. Anti cancer Activity:-
|
| |
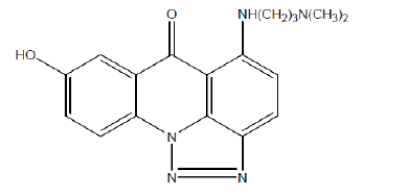
| Triazoloacridone (9) exhibits antitumour activity. It binds with DNA and induces structural modification.20 |
| |
 |
| |
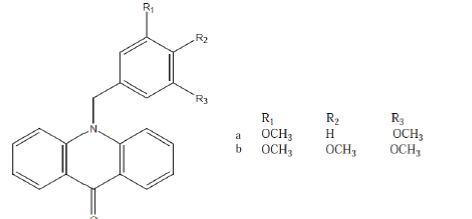
| Gao et al; (2008); synthesized a novel series of 10- benzyl-9(10H)-acridones (Compound 12) and 1- benzyl-4-piperidones and screened them for their invitro antitumour activities. The compounds a and b showed greater inhibitory effects.21 |
| |
 |
| |
| Neidle et al; (2007); presents the invention pertains generally to a class of compounds reffered as “acridones” and “acridines”. The ability of these compounds to inhibit the telomerase, to regulate the cell proliferation and in the treatment of proliferative conditions such as cancer was explained.22 |
| |
 |
| |
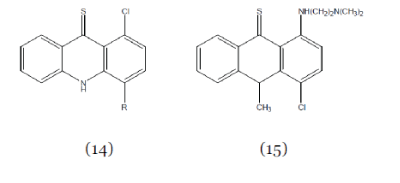
| Dheyongera et al; (2005); synthesized thioacridone compounds related to acronycine (Compound 14) and (Compound 15) and screened them for anticancer activity. The synthesized compounds in this series demonstrate good cytotoxic activity.23 |
| |
 |
| |
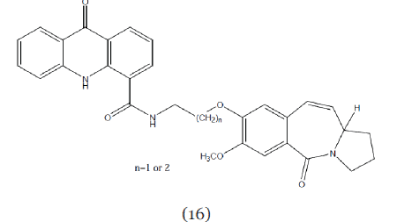
| Kamal et al; (2004);synthesized benzodiazepine hybrids linked to acridine/ acridone ring systems (Compound 16) at C-8 position. These derivatives showed promising in-vitro anticancer activity.24 |
| |
 |
| |
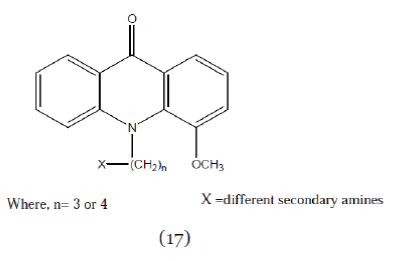
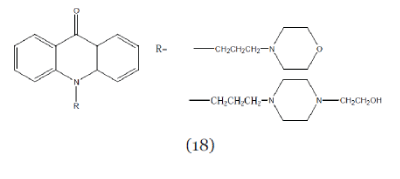
| Hedge et al; (2004); synthesized N10-substituted- 4-methoxyacridones (Compound 18) with different secondary amines. These compounds enhanced the uptake of vinblastin to greater extent than verapamil. The SAR studies revealed that substitution of -H at C-4 in acridone nucleus by –OCH3 increases the cytotoxicity and anti-MDR activity.25 |
| |
 |
| |
| Krishnegowda et al; (2002); synthesized a series of 19 N10-substituted methoxy acridine (Compound 18) analogue. All the synthesized compounds were screened for their ability toincrease the uptake of vinblastine and results showed that these compounds caused greateraccumulation of vinblastine than did a similar concentration of standard modulator verapamil.26 |
| |
 |
| |
|
Antileishmanial activity:-
|
| |
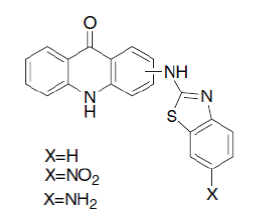
| Carole Di Giorgio et al; (2004); reported antileishmanial activity of (1,3-benzothiazol-2- yl)amino-9-(10H)-acridinone derivatives. Two derivatives, 4-(6-nitro-benzothiazol-2-ylamino)-10Hacridin- 9-one and 1-(6-amino-benzothiazole- 2ylamino)-10H-acridin-9-one revealed a selective antileishmanial activity.27 |
| |
 |
| |
| Various patents on acridone derivatives28-44: |
| |
 |
| |
ACKNOWLEDGEMENT:
|
| |
| The authors Palak K. Parikh and Hiren M. Marvaniya are thankful to the project guide Prof. Dr. Dhrubo Jyoti Sen and the staff members of Shri Sarvajanik Pharmacy College, Mehsana, Gujarat to fulfill the review article successfully with the help of search engine of the library of SSPC, Mehsana. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |


























