Keywords
Moringa oleifera, chemo protective, histopathological analysis, hepatic, renal
Introduction
Cancer is a complex disease attributed to the integrated outcome of carcinogen activation or detoxification, DNA repair capacity, and other known or unknown factors. Individual responses to a chemical carcinogenic agent depend on polymorphisms of enzymes responsible for metabolic activation /detoxification of the carcinogen, DNA repair, and apoptosis, as well as promotion and progression in malignantly transformed cells [1]. Certain toxic chemicals and medicines can cause hepatic and renal cancer, are recognized as a toxicological problem. The most important reason of these disorders is the exposure of different environmental pollutants and xenobiotics e.g., polyaromatic hydrocarban, thioacetamide, paracetamol, carbon tetrachloride, alcohol, etc. Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer mortality worldwide [2]. Hepatitis viral infections [3], toxic industrial chemicals, food additives, alcohol, fungal toxins (aflatoxins), air and water pollutants are the major risk factors of liver diseases [4,5]. Liver damage is always associated with cellular necrosis, increase in lipid peroxidation and depletion in the tissue GSH levels.
Kidney cancer is the third most common malignancy of the genitourinary system account for 2 to 3 % of all cancers in men worldwide, with 130,000 new cases and 63,000 deaths from the disease occurring annually [6]. Kidney injury or nephrotoxicity is characterized by tubular cell necrosis, which is localized mainly to the proximal tubule and renal atrophy [7]. Oxidative stress may alter the structure and function of the glomerulus because of the effect of ROS on mesangial and endothelial cells [8]. The glomerulus is considerably more sensitive to oxidative injuries than other nephron segments.
PAHs are widely distributed in our environment and are implicated in various types of cancer. The PAH 7, 12-dimethyl-benz[a]anthracene (DMBA) acts as a potent carcinogen by generating various reactive metabolic intermediates leading to oxidative stress [9, 10, 11]. It is therefore imperative to search alternative drugs for the treatment of liver disease to replace the currently used drugs of doubtful efficacy and safety [12]. DMBA-induced experimental carcinogenesis might therefore be used as an ideal model to study the chemopreventive potential of medicinal plants and their active constituents.
Medicinal plants, since time immemorial have been in use for treatment of various diseases all over the world [13]. Moringa oleifera Lam. (Syn Moringa pterygosperma Gaertn; Fam: Moringaceae) have been reported to known by regional names such as drumstick tree, sajiwan and sajna, is a natural as well as cultivated variety of the genus Moringa [14, 15]. Moringa oleifera possesses antitumor, antipyretic, antiepileptic, anti-inflammatory, antiulcer, antispasmodic, diuretic, antihypertensive, cholesterol lowering, antioxidant, antidiabetic, renal [16, 17] and hepatoprotective activities [18, 19]. Saponins have been found in many medicinal plants used in folk medicines [20, 21]. In this study, isolation of saponins was conducted from M. oleifera pods and further analysis was done to know anticarcinogenic potential of these isolated saponins. Saponins, by virtue of their multiple apoptotic actions on cancer cells, may provide a new line of anticancer agents. They are also effective against drug-resistant cancer cells [22]. To date, over hundreds of saponins have been described. However, given the diverse distribution of saponins, it can be conceived that a lot of novel anticancer saponins remain unexploited. Hence, the aim of this current investigation was to explore more the chemopreventive effects of the hydroethanolic pods extract of M. oleifera and effect of isolated saponin against environmental carcinogen DMBA by physiological and histopathological analysis.
Materials and Methods
Chemicals
DMBA was purchased from SIGMA chemical Co. (USA).
Procurement of plant material
The experimental plant Moringa oleifera was collected from Krishi Vigyan Kendra, Banasthali University, Banasthali, India, in the month of October 2009. The plant material was taxonomically identified by Botanist of Krishi Vigyan Kendra, Banasthali, Tonk district.
Soxhlet extraction of Plant Material: Direct extraction
For preparation of Hydro-ethanolic extract, dried powdered pods were placed in the Soxhlet thimble with 80% ethanol in 250 ml flat bottom flask. Collected solvent was cooled at room temperature and poured in a glass plate. The extract was concentrated under vacuum at 40 °C to yield a semisolid mass, dried in hot air oven below 50 °C for 48 hours and stored in a desiccator. The percentage yield of extract (MOHE) was found to be 22% and stored at 4 °C in airtight containers. Suspensions of the extract was prepared in distilled water and used to assess hepatic and renoprotective activity.
Sequential extraction of M. oleifera pods
The general procedure of saponin extraction was as follows: First, the plant material was defatted by petroleum ether. The powdered pods were then extracted with soxhlet apparatus using sequential solvents that was pet ether, benzene, chloroform, ethyl acetate than ethanol for 16 h. Aqueous extract was also obtained by soaking sequential plant part powder in double distilled water for 2-3 days and then filtered with cheese cloth. The extracts were than concentrated on a rotary evaporator below 50 °C and were stored in airtight containers in cold room or at 4 °C temperature for further studies.
Isolation and characterization of saponins
Thin layer chromatography (TLC) was carried out to isolate the principle components that were present in most effective extracts of plant. TLC study was carried out for different extracts. The solvent system for isolation of saponin used was: Chloroform: methanol: H2O (7:3:1). Characterization of isolated saponin was done using HPLC, IR and 1H NMR. The isolated compound was then assessed for its anticarcinogenic potential.
Experimental Animals
Male Swiss Albino mice (Mus musculus) weighing 15- 30 g were obtained from Haryana Agricultural University, Hissar (India) for experimental purpose. The animals were acclimatized for a month prior to experiment. The Institutional Animal Ethical Committee approved the animal studies. All experiments were conducted on adult male albino mice when they weighed 25-35 g (3-4 months old; twelve groups of six mice each). Colony bred adult male albino mice were maintained under standard laboratory conditions at a temperature of 22 ± 3 °C, relative humidity of 50 ± 5 % and photoperiod of 12h (dark and light cycle). The mice were housed in polypropylene cages. In order to avoid diurnal variation all the experiments were carried out at same time of the day. Animals lead free access to standard food pellet diet (Hindustan Lever Limited: metal contents in parts per million dry weight: Cu 10.0, Zn 45.0, Mn 55.0, Co 5.0, Fe 75.0) and drinking water ad libitum throughout the study.
Treatment Regime:
Adult Swiss albino male mice (Mus musculus L.) weighing 25-30 g were used for histopathological parameters. Mice (72) were divided into twelve groups of 6 mice each. The groups for each parameter were treated by oral gavage once, daily as follows:
Treatment consisted of pretreatment phase of MOHE in distilled water followed by the second phase in which the animals were given 15 mg/kg DMBA on day 22. The animals were sacrificed on 10th day after DMBA administration. The groups were as follows-
Group 1: served as control (normal untreated mice), and received 1ml distilled water daily by oral gavage.
Group 2: received distilled water for 21 days prior to a single dose of DMBA (15 mg/kg body weight: p.o) served as DMBA control group.
Group 3 and 4 were administered with hydroethanolic extract of MOHE pods (200 and 400 mg/kg body weight: p.o) daily for 21 days, served as MO treated control group.
Group 5 and 6: received BHA (0.5 % and 1%: p.o) daily for 21 days, dissolved in 0.5% acetone and served as standard treated control group.
Group 7: were administered with isolated saponin component of Moringa oleifera pods (SM; 50 mg/kg body weight: p.o) daily for 21 days, served as SM control group.
Group 8 and 9 were treated with hydro-ethanolic extract of MOHE pods (200 and 400 mg/kg body weight; p.o) daily for 21 days, before being intoxicated with DMBA (15 mg/kg body weight; p.o, once) dissolved in olive oil for 10 days.
Group 10 and 11: received BHA (0.5 % and 1%: p.o) daily for 21 days, before being intoxicated with DMBA (15 mg/kg body weight; p.o, once) dissolved in olive oil for 10 days.
Group 12: received isolated saponin component of Moringa oleifera pods (SM; 50 mg/kg body weight: p.o), before being intoxicated with DMBA (15 mg/kg body weight; p.o, once) dissolved in olive oil for 10 days.
All these groups of mice i.e. Group 2 to Group 12 served as treated groups and group 1 as a control group. DMBA was given after 21 days of plant extract administration. The mice were sacrificed after 10 days (depending on mortality of mice) of DMBA administration. Total duration of treatment for each group was of 31 days. After 31 days of duration the mice were fasted overnight and then on next day they were sacrificed under light chloroform anesthesia. The organs liver and kidneys, were dissected out, washed immediately, cleaned and rinsed in ice cold saline, blotted to remove blood, the wet weight noted and then stored at -80°C for histological studies.
Physiological Variables
Acute toxicity studies
Acute oral toxicity was performed as per OECD-423 guidelines [23]. The albino mice were fasted overnight provided only water, after which the hydro-ethanolic extract of the pods of MO was administered by gastric intubation to the relevant animals at the single dose of 15mg/kg body weight. The animals were then observed for 21 days. However, mortality was not observed, the procedure was repeated for further higher doses such as 50, 100, 150, 200, 300, 400, 800, 1600 mg/kg body weight. Mortality was not noticed up to 400 mg/kg, whereas, the LD50 of the extract was found to be 1800 mg/kg body weight. Toxic symptoms for which the animals were observed for 72 h include behavioural changes, locomotion, convulsions and mortality.
Body Weight and Organ Weight
The body weight of animals recorded at the end of the experiment was nearest to gram. The absolute weight of organ (liver and kidney) for different groups of experimental animals was recorded to the nearest milligram on a microbalance.
The Relative organ weight or somatic index was calculated which is the ratio of organ weight to final body weight [12].
Histopathological Studies
Portion of mice liver and kidney were collected and histopathological analysis was done by method of Manus and Mowry [24].
Statistical Analysis:
The experimental results obtained are expressed as mean ± standard error (SEM) of three replicates. The data was subjected to one way analysis of variance (ANOVA) and differences between samples were determined by Tukey multiple comparison test using the SPSS 16.0 (Statistical program for Social Sciences) program. The level of significance was set at p<0.001.
Experimental Results
The outcome of this study supports in in-vivo protective potential of Moringa oleifera pods extract (MOHE) and its isolated saponin (SM) during DMBA exposure. All animals survived during entire experimental period. A marked behavioral distinction was observed in the animals treated with DMBA only, which were comparatively very active and more irritable with respect to control groups. DMBA treatment at the stipulated dose and duration resulted in body hair loss and loose skin and irritation. The physiological results obtained are tabulated and depicted below:-
Compound isolation and characterization
Thin Layer Chromatography (TLC) of all sequential extracts of Moringa oleifera pods obtained by sequential extraction methods was carried out to confirm its nature by analyzing TLC chromatograms and to isolate active saponin ingredients from the extracts.
TLC of benzene extract of Moringa oleifera pods revealed the presence of 8 compounds (corresponding to 8 spots) when a solvent phase of chloroform: methanol: H2O (7:3:1) is used. Compounds having Rf of 0.90 and 0.87 were most prominent and clear spots (green spots). The first spot is chosen for the study and is nomenclature as SM (isolated saponin). Characterization of isolated saponin was done using HPLC, IR and 1H NMR.
Acute toxicity studies
There were no adverse effects of MOHE on the animals at the given dose levels (200 and 400mg/ kg body weight/day for 21 days respectively). Acute toxicity studies indicated that hydro-ethanolic extract of Moringa oleifera pods was found to be non-toxic up to dose of 900mg/ kg body weight. The hepato and renoprotective effect offered by 400mg/ kg body weight was found to be greater than that of 200mg/kg body weight. BHA (0.5% and 1%) was used as a positive control in the present study.
Effect of plant extract, BHA and isolated SM on bodyweight of mice
Bodyweight was decreased significantly from 25.06±1.19g to 20.13±1.38 g (P<0.001), on 10th day during DMBA treatment as compared to control group. Groups of mice which received plant extract, BHA and isolated SM alone (gp 3 to gp 7) showed bodyweight comparable to control at all time interval, except in MOHE (400) receiving group on 21st day which showed significant increase (P<0.001) in bodyweight as compared to control. Pre-treatment of mice with plant extract, BHA and SM before DMBA intoxication (gp 8 to gp 12) showed significant elevation in bodyweight (P<0.001) from 24.62±1.17 g to 28.12±1.04 g (group 8), 24.32±1.13 g to 31.02±1.11 g (group 9), 24.58±1.14 g to 25.12±1.25 g (group 10), 24.28±1.36 g to 27.12±0.75 (group 11) g, 24.16±1.14 g to 31.12±0.15 (group 12), respectively on 31st day as compared to DMBA received groups of mice (table 1).
| Groups |
Treatme nts (mg/kg) |
Initial Weight (g) |
Final Weight (g) |
| CT (1) |
- |
25.52 ± 1.14 |
27.98 ± 1.52 |
| DMBA (2) |
15 |
25.06 ± 1.19a |
20.13 ± 1.38a |
| MO 200 (3) |
200 |
24.17 ± 1.52 |
25.96 ± 0.16 |
| MO 400 (4) |
400 |
24.24 ± 0.92a |
31.10 ± 0.82a |
| BHA (5) |
0.5% |
24.16 ± 0.87 |
25.50 ± 0.99 |
| BHA (6) |
1% |
24.12 ± 1.23 |
24.92 ± 1.52 |
| SM (7) |
50 |
24.18 ± 1.11 |
26.17 ± 1.08 |
| MO + DMBA (8) |
200+15 |
24.62 ± 1.17* |
28.12 ± 1.04* |
| MO + DMBA (9) |
400+15 |
24.32 ± 1.13* |
31.02 ± 1.11* |
| BHA + DMBA (10) |
0.5%+15 |
24.58 ± 1.14* |
25.12 ± 1.25* |
| BHA + DMBA (11) |
1%+15 |
24.28 ± 1.36* |
27.12 ± 0.75* |
| SM + DMBA (12) |
50+15 |
24.16 ± 1.14* |
31.12 ± 0.15* |
Values are expressed as Mean ± SEM (n=6). ap<0.001 vs. control group; *p<0.001 vs Treated (DMBA) group
Table 1: Effect of plant extract, BHA and isolated SM on bodyweight of DMBA intoxicated mice
Effect of plant extract, BHA and isolated SM on liver weight in mice
Liver weight was increased significantly (P<0.001) during DMBA treatment as compared to control group. Groups of mice that received plant extract, BHA and isolated SM alone showed liver weight comparable to control at all time interval. Pretreatment of mice with plant extract, BHA and SM before DMBA intoxication showed significant depletion in liver weight (P<0.001) respectively on 31st day as compared to DMBA treated group (table 2), restoring back the values towards normal. No significant increase or decrease in liver weight was observed in mice that received doses of BHA along with DMBA.
| Groups |
Treatments (mg/kg) |
LIVER (g) |
RELATIVE WEIGHT % |
KIDNEY (g) |
RELATIVE WEIGHT % |
| CT (1) |
- |
1.78 ± 0.21 |
6.36 |
0.53 ± 0.18 |
1.89 |
| DMBA (2) |
15 |
2.35 ± 0.43a |
11.67 |
0.70 ± 0.23a |
3.47 |
| MO 200 (3) |
200 |
1.69 ± 0.98 |
6.51 |
0.47 ± 0.12 |
1.81 |
| MO 400 (4) |
400 |
1.94 ± 1.26 |
6.23 |
0.55 ± 0.23 |
1.76 |
| BHA (5) |
0.5% |
1.75 ± 1.53 |
6.86 |
0.52 ± 0.32 |
2.03 |
| BHA (6) |
1% |
1.77 ± 1.21 |
7.10 |
0.49 ± 0.31 |
1.96 |
| SM (7) |
50 |
1.85 ± 0.86 |
7.06 |
0.62 ± 0.27 |
2.36 |
| MO + DMBA (8) |
200+15 |
2.05 ± 0.19* |
7.29 |
0.53 ± 0.15* |
1.88 |
| MO + DMBA (9) |
400+15 |
2.25 ± 0.37* |
7.25 |
0.65 ± 0.15* |
2.09 |
| BHA + DMBA (10) |
0.5%+15 |
1.98 ± 0.28* |
7.88 |
0.57 ± 0.19* |
2.26 |
| BHA + DMBA (11) |
1%+15 |
2.05 ± 0.06* |
7.55 |
0.45 ± 0.21* |
1.65 |
| SM + DMBA (12) |
50+15 |
2.09 ± 0.17* |
6.71 |
0.64 ± 0.15* |
2.05 |
Values are expressed as Mean ± SEM (n=6). ap<0.001 vs. control group; *p<0.001 vs Treated (DMBA) group
Table 2: Effect of plant extract, BHA and isolated SM on organ weight (liver and kidney) of DMBA intoxicated mice
Effect of plant extract, BHA and isolated SM on kidney weight in mice
Groups of mice, which were administered with DMBA alone (gp 2), showed significant increase (P<0.001) in kidney weight during DMBA treatment as compared to control group 1. Groups of mice that received plant extract, BHA and isolated SM alone showed almost no significant increase or decrease in kidney weight at all time interval. Pre-treatment of mice with plant extract, BHA and SM before DMBA intoxication showed significant decline (P<0.001) in kidney weight respectively on 31st day as compared to DMBA treated group (table 2), restoring back the values towards normal. No significant increase or decrease in kidney weight was observed in mice that received doses of BHA along with DMBA.
Histopathological Analysis
Liver
The histological examinations basically support the results obtained from antioxidant enzymes and tumor marker assays. Histopathology of control mice (group 1) which received normal saline, showed normal architecture and cells cytoplasm of hepatic cells with granulated cytoplasm, central vein, small uniform nuclei of liver cell on 31st days of study (Figure 1.). DMBA-treated mice (group 2) showed loss of architecture and neoplastic cells arranged in lobules separated by fibrous septa with inflammatory collection and small bile duct proliferation. Neoplastic cells were smaller than normal cells with granular cytoplasm and larger hyperchromatic nuclei and tumor cells also contain intracytoplasmic violaceous, hyaline globules that represent proteins produced by the tumor cells, nuclear condensation in Periportal zone and liver cell bearing hepatocellular carcinoma on 31st day of study (Figure 2.). Liver section of mice which received MOHE extract at both doses (200 and 400mg/kg bw), standard BHA (0.5 and 1%) and isolated SM (50mg/kg bw) only (group 3-7) showed normal histological appearance of liver cells with some hepatocytes and an isokaryosis minimal inflammatory cell infiltration around the portal triads (Figure 3-Figure 7.).
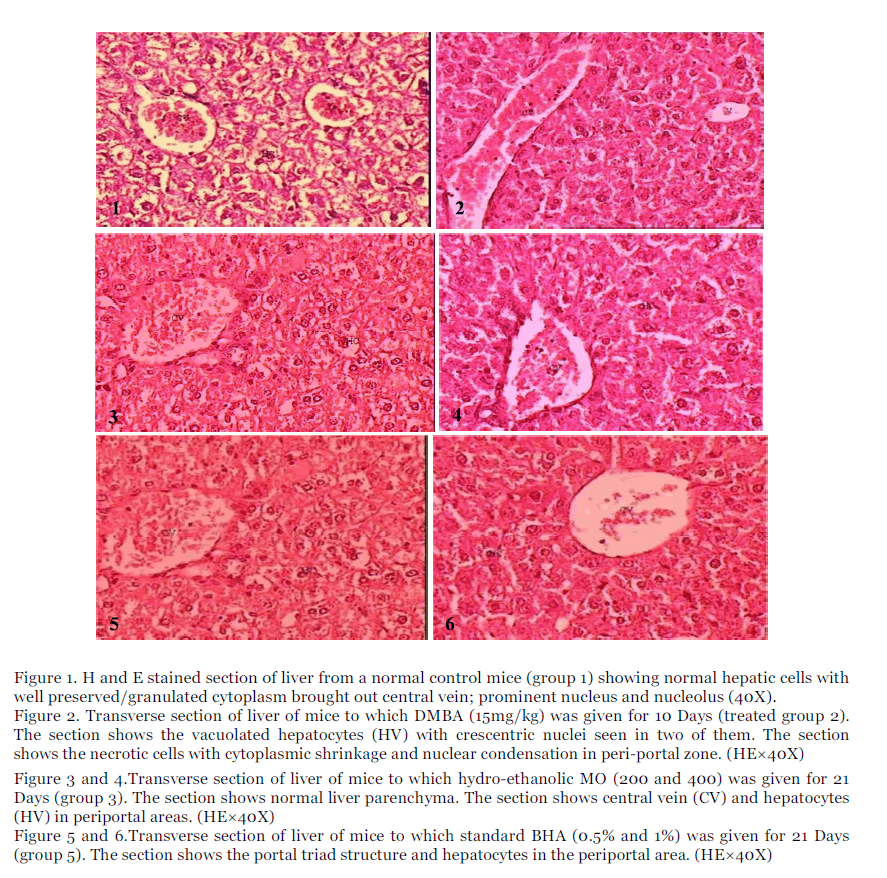
Figure 1. H and E stained section of liver from a normal control mice (group 1) showing normal hepatic cells with well preserved/granulated cytoplasm brought out central vein; prominent nucleus and nucleolus (40X).
Figure 2. Transverse section of liver of mice to which DMBA (15mg/kg) was given for 10 Days (treated group 2). The section shows the vacuolated hepatocytes (HV) with crescentric nuclei seen in two of them. The section shows the necrotic cells with cytoplasmic shrinkage and nuclear condensation in peri-portal zone. (HE×40X)
Figure 3 and 4.Transverse section of liver of mice to which hydro-ethanolic MO (200 and 400) was given for 21 Days (group 3). The section shows normal liver parenchyma. The section shows central vein (CV) and hepatocytes (HV) in periportal areas. (HE×40X)
Figure 5 and 6.Transverse section of liver of mice to which standard BHA (0.5% and 1%) was given for 21 Days (group 5). The section shows the portal triad structure and hepatocytes in the periportal area. (HE×40X)
Section of mice which received MOHE 200 before DMBA intoxication (group 8) showed swelling of hepatocytes throughout the liver lobules, hepatocytes with double nuclei but normal cytoplasm on 31st day where as MOHE 400 before DMBA challenge (group 9) showed normal hepatocytes in Periportal zone on 31st day (Figure 8 and Figure 9), whereas mice which received BHA 0.5% before DMBA challenge (group 10) showed degeneration of hepatocytes in liver periportal zone, scattered and vacuolated epithelium in mid zone (Figure 10) and BHA 1% before DMBA administeration (group 11) showed cattered vacuolated hepatocytes in the mid and periportal zones along with expanded central vein (Figure 11) SM before DMBA administration (group 12) showed normal hepatocytes in Periportal zone on 31st day (Figure 12).
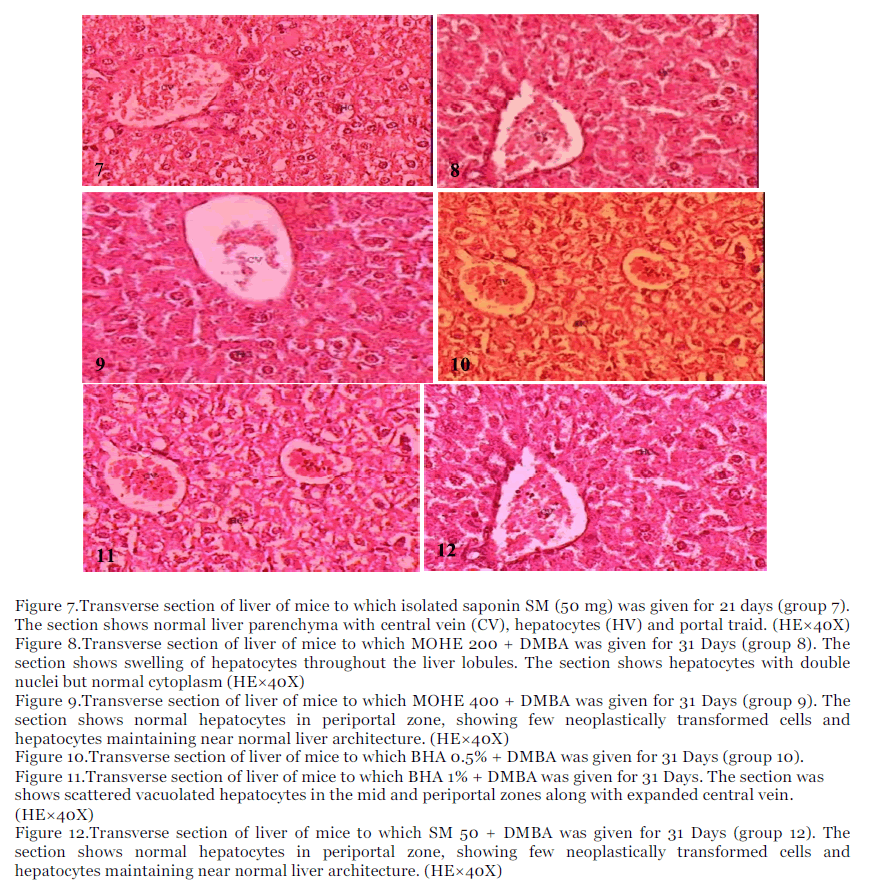
Figure 7.Transverse section of liver of mice to which isolated saponin SM (50 mg) was given for 21 days (group 7). The section shows normal liver parenchyma with central vein (CV), hepatocytes (HV) and portal traid. (HE×40X)
Figure 8.Transverse section of liver of mice to which MOHE 200 + DMBA was given for 31 Days (group 8). The section shows swelling of hepatocytes throughout the liver lobules. The section shows hepatocytes with double nuclei but normal cytoplasm (HE×40X)
Figure 9.Transverse section of liver of mice to which MOHE 400 + DMBA was given for 31 Days (group 9). The section shows normal hepatocytes in periportal zone, showing few neoplastically transformed cells and hepatocytes maintaining near normal liver architecture. (HE×40X)
Figure 10.Transverse section of liver of mice to which BHA 0.5% + DMBA was given for 31 Days (group 10).
Figure 11.Transverse section of liver of mice to which BHA 1% + DMBA was given for 31 Days. The section was shows scattered vacuolated hepatocytes in the mid and periportal zones along with expanded central vein. (HE×40X)
Figure 12.Transverse section of liver of mice to which SM 50 + DMBA was given for 31 Days (group 12). The section shows normal hepatocytes in periportal zone, showing few neoplastically transformed cells and hepatocytes maintaining near normal liver architecture. (HE×40X)
II. Kidney
Histopathology of control mice (group 1) showed cortical region of normal kidney with glomerulus and tubules (Figure 13). While section of mice kidney treated with DMBA (group 2) showed vacuolar degeneration of tubular epithelial, flattening of tubular epithelial cell with disappearance of brush border and presence of cast in tubular lumen. The glomerulus showed congestion in RBC and vacuolation, necrosis and shedding of tubular epithelial cells on 31st day (Figure 14). Kidney section of mice which received MO extracts at both doses (200 and 400mg/kg), standard BHA (0.5 and 1%) and isolated SM (50mg/kg bw) only (group 3-7) showed normal architecture of kidney cells (Figure 15-Figure 19.).
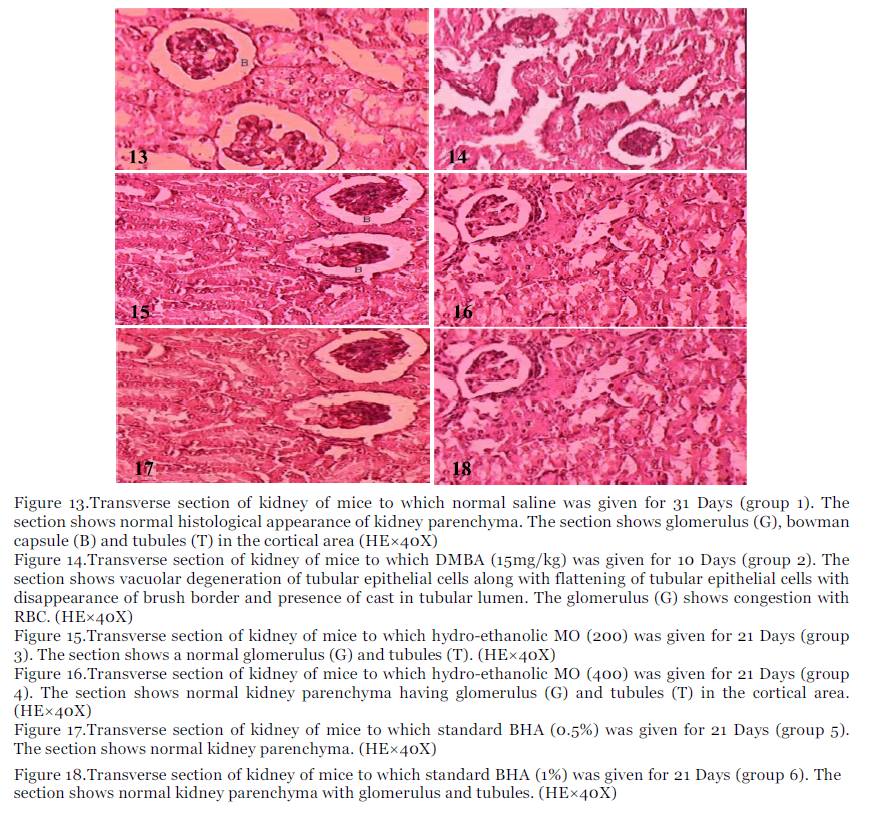
Figure 13.Transverse section of kidney of mice to which normal saline was given for 31 Days (group 1). The section shows normal histological appearance of kidney parenchyma. The section shows glomerulus (G), bowman capsule (B) and tubules (T) in the cortical area (HE×40X)
Figure 14.Transverse section of kidney of mice to which DMBA (15mg/kg) was given for 10 Days (group 2). The section shows vacuolar degeneration of tubular epithelial cells along with flattening of tubular epithelial cells with disappearance of brush border and presence of cast in tubular lumen. The glomerulus (G) shows congestion with RBC. (HE×40X)
Figure 15.Transverse section of kidney of mice to which hydro-ethanolic MO (200) was given for 21 Days (group 3). The section shows a normal glomerulus (G) and tubules (T). (HE×40X)
Figure 16.Transverse section of kidney of mice to which hydro-ethanolic MO (400) was given for 21 Days (group 4). The section shows normal kidney parenchyma having glomerulus (G) and tubules (T) in the cortical area. (HE×40X)
Figure 17.Transverse section of kidney of mice to which standard BHA (0.5%) was given for 21 Days (group 5). The section shows normal kidney parenchyma. (HE×40X)
Figure 18.Transverse section of kidney of mice to which standard BHA (1%) was given for 21 Days (group 6). The section shows normal kidney parenchyma with glomerulus and tubules. (HE×40X)
Section of mice which received MOHE 200 before DMBA challenge (group 8) showed congested glomerulus and tubules with vacuolated epithelial cells, vacuolation of tubular epithelium in one tubule with normal lying adjacent to it on 31st day (Figure 20) where as MOHE 400 before DMBA administration (group 9) showed normal renal parenchyma on 31st day (Figure 21) Whereas mice which received BHA 0.5% before DMBA (group 10) showed normal tubules with congested glomerulus and normal epithelium 31st day (Figure 22) and BHA 1% (group 11) showed vacuolation of tubular epithelium but no shedding of tubular cells into the lumen tubes with fattening of tubular epithelial cells but no vacuolization or shedding of cells and showed normal renal parenchyma cell on 31st day (Figure 23), where as SM before DMBA administration (group 12) showed normal renal parenchyma on 31st day (Figure 24).
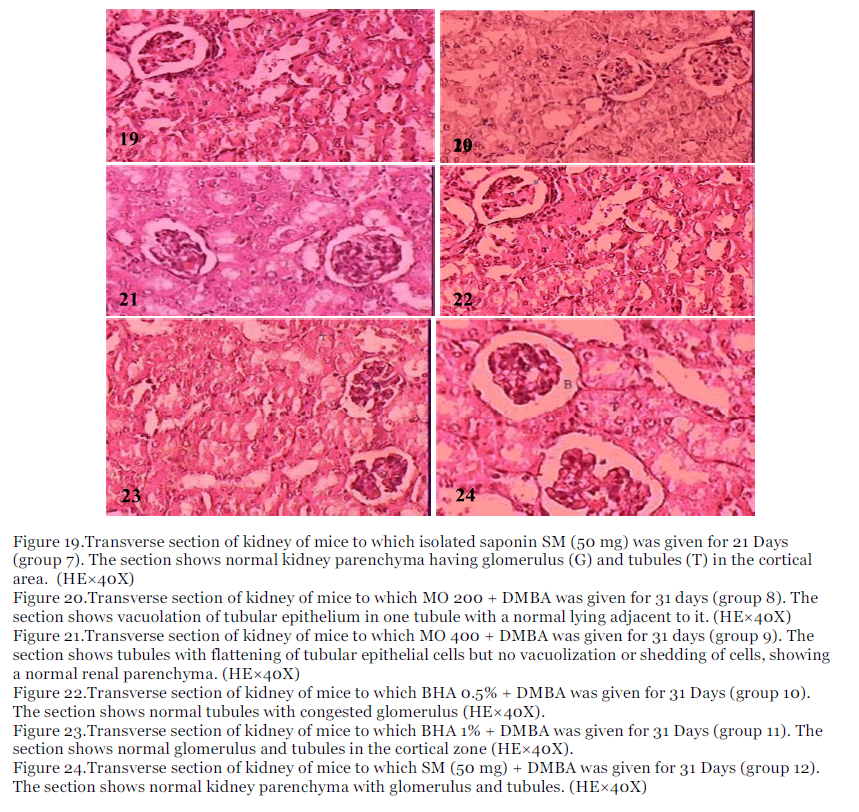
Figure 19.Transverse section of kidney of mice to which isolated saponin SM (50 mg) was given for 21 Days (group 7). The section shows normal kidney parenchyma having glomerulus (G) and tubules (T) in the cortical area. (HE×40X)
Figure 20.Transverse section of kidney of mice to which MO 200 + DMBA was given for 31 days (group 8). The section shows vacuolation of tubular epithelium in one tubule with a normal lying adjacent to it. (HE×40X)
Figure 21.Transverse section of kidney of mice to which MO 400 + DMBA was given for 31 days (group 9). The section shows tubules with flattening of tubular epithelial cells but no vacuolization or shedding of cells, showing a normal renal parenchyma. (HE×40X)
Figure 22.Transverse section of kidney of mice to which BHA 0.5% + DMBA was given for 31 Days (group 10). The section shows normal tubules with congested glomerulus (HE×40X).
Figure 23.Transverse section of kidney of mice to which BHA 1% + DMBA was given for 31 Days (group 11). The section shows normal glomerulus and tubules in the cortical zone (HE×40X).
Figure 24.Transverse section of kidney of mice to which SM (50 mg) + DMBA was given for 31 Days (group 12). The section shows normal kidney parenchyma with glomerulus and tubules. (HE×40X)
Discussion
The use of Medicinal plants as anticancer agents has a long history that began with folk medicine and several drugs currently used in chemotherapy are isolated from plant species or it is derived from plant products [25, 26, 27]. Recently considerable attention has been focused on identifying naturally occurring chemo preventive agents capable of inhibiting, retarding or reversing multistage carcinogenesis [28, 29]. The liver and kidney of tumor bearing animal has evolved as a reliable model for studying malignant transformation and interventions by chemopreventive agents [30]. The body weight and organ weight of treated animals remained comparable to control indicating a favourable effect of the modulator on general body metabolism. These results are in consistent with Bharali et al [12].
The effect of DMBA on liver cells has been widely studied by both at the histological and the ultrastructural level. In the present investigation, DMBA exposure produced pronounced hepatic histopathology evidenced by histological alternations in liver include focal necrosis with hepatocyte vacuolation dilation of central vein and sinusoids. These findings are in support with Vijayabaskaran et al. [31]. DMBA hepatotoxicity leads to vacuolization of the cells, polymorphism of the nuclei and a decrease in glycogen content of the hepatocytes [31]. The pathological changes may lead to impaired liver function, which interferes with the secretion of plasma proteins and also showed lymphoblastic cells in the liver or lymphosarcoma. These findings are in support with Hamid et al. [32]. Organ pathology showed that no significant lesions were observed in this study and this may point to the fact that this plant is relatively safe for use nutritionally and medicinally [33]. These pathological changes were prevented to moderate extent in Moringa oleifera and isolated saponin from pods extracts treated groups. This might be due to the presence of saponins, flavonoids and phenolics [20, 21]. Antioxidant property is claimed to be one of the mechanism of hepatoprotective drugs [34]. Further saponins, flavonoids and polyphenols have been suggested to act as antioxidants by free radical scavenging [35, 36]. Thus the hepatoprotective activity of Moringa oleifera may be attributed due to substances including antioxidants, flavonoids, saponins and other substances [20, 21]. Finally, these results suggested that Moringa extract can act against DMBA-induced liver injury and fibrosis in mice by a mechanism related to its antioxidant properties, antiinflammatory effect and its ability to attenuate the hepatic stellate cells activation [37].
Effect on histology of renal tissue
From the results of current study, DMBA exposure produced marked histological alternations in kidney include dilation of tubules; sloughing of epithelium indicates advanced disintegration of tubules. These results suggest that the kidney may be a major target organ of DMBA toxicity, after mammary glands, skin and liver and that the epithelial cells of proximal convoluted tubules and Bowman’s capsule seem to be more sensitive to DMBA induced nephrotoxicity. Many recent studies have provided experimental evidences that DMBA exposure can result in the generation of ROS and cause cell damage or death through the ROS signaling pathway [12, 21, 38]. The results of the present work showed that the tubular damages were more prominent in the proximal convoluted tubules in comparison to that in the distal ones. This could be due to the fact that the proximal convoluted tubules are the primary sites of reabsorption and active transport leading to higher concentration of DMBA in the epithelial lining of these tubules. Organ pathology showed that no significant lesions were observed in this study and this may point to the fact that this plant is relatively safe for use nutritionally and medicinally [33]. These pathological changes were prevented to moderate extent in Moringa oleifera and isolated saponin from pods extracts treated groups. This might be due to the presence of saponins, flavonoids and phenolics [20, 21]. Kidney of mice ingested to Moringa oleifera and isolated saponin from pods extracts showed the tubules appear more or less normal. The chemical constituents like vitamin A, nicotinic acid, ascorbic acid, vitamin B, fatty acid, glucose, sucrose, citric acid, malic acid, succinic acid, fumaric acid and oxalic acid present in Moringa oleifera extract produced protective effects in renal tissue against DMBA toxicity [39, 40].
Conclusion
In conclusion the current study suggests that hydro-ethanolic ethanolic extract of Moringa oleifera and its isolated saponin can prevent or slow down the oxidative damage induced by DMBA in mice. These novel findings suggest that M. oleifera exerts its chemo-preventive effects by enhancing the levels of antioxidants in DMBA carcinogenesis by reducing the formation of free radicals. The pathological changes in liver were prevented to moderate extent in Moringa oleifera treated groups. Kidney of mice ingested to Moringa oleifera and DMBA extracts shows the tubules appear more or less normal. Thus Moringa oleifera produced protective effects in hepatic and renal tissue against DMBA toxicity. This study rationalized the ethno-medicinal use of M. oleifera for protection against renal-toxicity induced by chemical carcinogens.
Acknowledgments
The authors are grateful to University Grants Commission (UGC) for providing financial assistance. The authors are thankful to the authorities of Banasthali University for providing support to the study.
Conflict of Interest
None.
Source of Support
NONE
5138
References
- Klaunig JE, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol and Applied Pharmacol. 2011; 254: 86–99.
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2005; 55: 74–105.
- Schütte K, Bornschein J, Malfertheiner P. Hepato cellular carcinoma-epidemiological trends and risk factors. Dig Dis. 2009; 27: 80–92.
- Farazi1 PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006, 6: 674-687.
- Jemal A, Siegel R, Ward E, Murray T, Xu JQ, Thun MJ. Cancer Statistics. CA Cancer J Clin, 2007, 57: 43-66.
- Janmeda P, Sharma V, Singh L, Paliwal R, Yadav S, Sharma SH. Chemopreventive effect of hydroethanolic extract of Euphorbia neriifolia leaves against DENA-induced renal carcinogenesis in mice. Asian Pacific J Cancer Prev, 2011; 12: 677- 83.
- Dehghani F, Nanawar MR, Noorfshan A, Karbalay- Doust S, Esmaeilpour T. Evaluation of the kidney extract on gentamicin induced-nephrotoxicity in rats. Kidney Res. 2011; J (1): 24-32.
- Kandeel M, Abdelaziz I, Elhabashy N, Hegazy H, Tolba Y. Nephrotoxicity and Oxidative Stress of Single Large Dose or Two Divided Doses of Gentamicin in Rats. Pak J of Biol Sci. 2011; 14: 627- 633.
- IARC. Some Polycyclic Aromatic Hydrocarbons and Heterocyclic Compounds. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, Lyon, France: International Agency for Research on Cancer. 1973; 3: 271.
- IARC. Polynuclear Aromatic Compounds, Part 1. Chemical, Environmental and Experimental Data. IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Humans, Lyon, France: International Agency for Research on Cancer. 1983; 32.
- IARC. Overall Evaluations of Carcinogenicity. IARC Monographs on the Evaluation of carcinogenic risk of chemicals to humans, supplement 7. Lyon, France: International Agency for Research on Cancer. 1987; 7: 440.
- Bharali R, Tabassum J, Azad MRH. Chemomodulatory effect of Moringa oleifera, Lam. on hepatic carcinogen metabolizing enzymes, antioxidant parameters and skin papillomagenesis in mice. Asian Pacific J. of Cancer Prevention. 2003; 4: 131-139.
- Satyanarayana K. Chemical examination of Scoparia dulcis (Linn). J of the Ind Chem Soc. 1969; 46: 765-766.
- Ramachandran C, Peter KV, Gopalakrishnan PK. Drumstick (Moringa oleifera): A multipurpose Indian Vegetable. Economic Botany. 1980; 34(3): 276-283.
- Paliwal R, Sharma V, Pracheta. A review on horse radish tree (Moringa oleifera): A multipurpose tree with high economic and commercial importance. Asian J. Biotechnol. 2011a; 3(4): 317-328.
- Paliwal R, Sharma V, Pracheta, Sharma S, Yadav S, Sharma SH. Antinephrotoxic effect of administration of Moringa oleifera Lam in amelioration of DMBA-induced renal carcinogenesis in Swiss albino mice. Biol Med. 2011b; 3(2): 27-35.
- Sharma V, Paliwal R, Janmeda P, Sharma SH. Renoprotective effects of Moringa oleifera pods in 7, 12-dimethylbenz[a]anthracene exposed mice. J of Chinese Int. Med. 2012a, 10 (10): 1171-1178.
- Paliwal R, Sharma V, Pracheta, Sharma SH. Hepatoprotective and antioxidant potential of Moringa oleifera pods against DMBA-induced hepatocarcinogenesis in male mice. Int J of Drug Dev and Res. 2011c; 3(2): 128-138.
- Sharma V, Paliwal R, Janmeda P, Sharma SH. Chemopreventive Efficacy of Moringa oleifera Pods Against 7, 12-dimethylbenz[a]anthracene Induced Hepatic Carcinogenesis in Mice. Asian Pacifc J Cancer Prev. 2012b; 13: 2563-2569.
- Paliwal R, Sharma V, Pracheta, Sharma S. Elucidation of free radical scavenging and antioxidant activity of aqueous and hydro-ethanolic extracts of Moringa oleifera pods. Res J of Pharm and Technol. 2011d; 4(4): 566-571.
- Sharma V, Paliwal R, Pracheta, Sharma S. Phytochemical analysis and evaluation of antioxidant activities of hydro-ethanolic extracts of Moringa oleifera lam. Pods. J of Pharm Res. 2011; 4(2): 554-557.
- Cheung JYN, Ong RCY, Suen YK, Ooi V, Wong HNC, Mak TCW, Fung KP, Yu B, Kong SK. Polyphyllin D is a potent apoptosis inducer in drug- resistant HepG2 cells. Cancer Lett. 2005; 217: 203- 211.
- Ecobichon DJ. The basis of toxicology testing. 2nd ed. CRC Press, New York. 1997: 43-60.
- Mc Manus and Mowry WR. Staining methods, Harper and Row, New York, 1965.
- Cragg GM, Boyd MR, Cardellina JH, Newman DJ, Snader KM, McCloud TG. Ethnobotany and drug discovery experience of the US National Cancer Institute. In: Chadwick, D.J., Marsh, J.(Eds.), Ciba Foundation ìEthnobotany and the search for new drugsîSymposium, 185. Wiley and Sons, Chichester, 1994, UK, pp. 178ñ196.
- Wang HK. Plant-derived anticancer agents currently in clinical use or clinical trials. The Investigation Drugs Journal. 1998; 1: 92ñ102.
- Cragg GM, Newman DJ. Antineoplastic agents from natural sources: achievements and future directions. Expert Opinion on Investigational Drugs .2000; 9: 1ñ15.
- Morse MA, Stoner GD. Cancer chemoprevention: principles and prospects. Carcinogenesis. 1993; 14: 1737ñ1746.
- Kelloff GJ, Hawk ET, Crowell JA, Boone CW, Nayfield SG, Perloff M, Steele VE, Lubet, Sigman. Strategies for identification and clinical evaluation of promising chemopreventive agents. Oncology.1996; 10: 1471ñ 1480.
- Balasenthil S, Nagini. Garlic exerts hepatoprotective effects during 4-nitroquinoline 1- oxide induced oral carcinogenesis in rats. Asia pacific J Clin Nutr. 2000; 9(2): 136-138.
- Vijayabaskaran M, Yuvaraja KR, Babu G, Sivakumar P, Perumal P, Jayakar B. Hepatoprotective and antioxidant activity of Symplocos racemosa bark extract on DMBA induced hepatocellular carcinoma in rats. Inter J Curr Trends Sci Tech. 2010; 1(3): 147-158.
- Hamid IS, Sugiyanto, Meiyanto E, Widyarini S. CYP1A1 and GSTµ expression of hepatocytes induced by 7,12-dimethylbenz(a)anthracene and the influence of Ethanolic Extract of Gynura procumbens, Indo J Pharm. 2009;20(4) : 198-206.
- Adedapo AA, Mogbojuri OM and Emikpe BO. Safety evaluations of the aqueous extract of the leaves of Moringa oleifera in rats. J of Med Plants Res. 2009; 3(8): 586-591.
- Fakurazi S, Hairuszah I, Nanthini U. Moringa oleifera Lam prevents acetaminophen induced liver injury through restoration of glutathione level. Food and Chemical Toxicol. 2008; 46: 2611–5.
- Kumar A, Pari L. Antioxidant action of Moringa oleifera Lam (drumstick) against antitubercular drugs induced lipid peroxidation in rats. J.Med.Food. 2003; 6: 255–259.
- Arabshahi DS, Devi V and Urooj A. Evaluation of antioxidant activity of some plant extracts and their heat, pH and storage stability. Food Chem. 2007; 100: 1100–1105.
- Hazma AA. Ameliorative effects of Moringa oleifera Lam seed extract on liver fibrosis in rats. Food and Chemical Toxicol. 2010; 48: 345-355.
- Parmar J, Sharma P, Verma P, Sharma P, Goyal PK. Modulation of DMBA induced biochemical and histopathological changes by Syzygium cumini seed extract during skin carcinogenesis. Int J Cur Biomed Phar Res. 2011; 1(2): 24-30.
- Ranjan R, Swarup D, Patra RC, Chandra, Vikas. Tamarindus indica L. and Moringa oleifera M. Extract administration ameliorates fluoride toxicity in rabbits. Ind J of Experimental Biol, 2009; 47 (11): 900-905.
- Pydi R, Rajalakshmi I, Indumathy S, Kavimani S. Nephroprotective medicinal plants -A review. Int J of Universal Pharmacy and Life Sci. 2011; 1(2): 1-16










