Keywords
Chlamydia trachomatis; Rheumatoid arthritis; Amyloidosis
Introduction
The role of polymorphism of the gene encoding the precursor protein in secondary amyloidosis - serum amyloid A (SAA) has been actively discussed for decades [1]. It has been shown that Japanese patients with rheumatoid arthritis having the SAA γ/γ genotype have a higher risk of developing amyloidosis [2,3]. In a few studies involving European patients with RA, the development of this complication was associated with the SAA α/α genotype [4]. We also established a high incidence of this variant of the genotype in patients with rheumatoid arthritis and secondary amyloidosis from Belarus [5].
Therefore, one can suppose that Chlamydia trachomatis infection can be a risk factor of secondary amyloidosis development in RA patients. We observed a number of cases when secondary amyloidosis developed in RA patient with concomitant C. trachomatis infection. The choice of this pathogen is not accidental. Chlamydia trachomatis may persist in the host organism for a long time, they are of low immunogenicity and widely spread (about 90 million novel contaminations per year), including RA patients [6]. Besides, it impacts the clinical manifestations of RA, so we have an opportunity to reveal subjects suspicious to C. trachomatis infection from entire RA patient’s cohort. SAA (Serum amyloid A)-the precursor protein in secondary amyloidosis-is similar to C-reactive protein (CRP) and is synthesized by liver under inflammatory condition. SAA level significantly increases in inflammation, as well as in chronic infection. Proinflammatory cytokines (interleukin-1, interleukin-6, tumor necrosis factor-α, etc.) induce activity of serum amyloid A gene and, consequently, increase SAA synthesis [7,8]. In normal conditions SAA level is less than 10 μg/ml [9,10]. The concentration of SAA increases in 1000-folder during inflammation. Correlation between SAA levels and risk of secondary amyloidosis development is contradictive [11-14]. Some authors consider SAA to be a survival predictor in patients with RA complicated by secondary amyloidosis [15]. Deposition of AA amyloid is considered to slow down when SAA concentration less than 3 μg/ml [16,17]. In its turn, SAA induces formation of insoluble derivatives in tissue that leads to progression of ÃÂÂÂÂÂÂÂÃÂÂÂÂÂÂÂ-amyloidosis.
In clinical practice we commonly observe combination of rheumatoid arthritis, mostly seronegative, with persistent Chlamydia trachomatis infection. Therefore, one can suppose, that the presence of persistent C. trachomatis infection in patient with rheumatoid arthritis may act as a supplementary stimulus for the development of secondary amyloidosis.
Now-a-days the majority of authors ranks Chlamydia as bacterium [18]. In contrast to viruses’ Chlamydia trachomatis possesses both DNA and RNA, as well as independent nucleic acid synthesis [19]. It has sensibility both to tetracycline and macrolide antibiotics, it possesses folic acid synthesis enzymes. However, in its development cycle Chlamydia trachomatis has much in common with viruses (in an early stage of development) as well as with cell organisms. It is well known that Chlamydia possesses unique intracellular development cycle, which allows concerning it as a separate family Chlamidiaceae.
In its life cycle Chlamydia trachomatis may exist in two forms-elementary bodies (EB) and reticular bodies (RB) [20]. As EB C. trachomatis can be found both intracellular and extracellular (in exudates). In this form it shows contagious properties, non-active to antigen stimuli, storage stable, insensitive to antibiotics action in vitro and able to penetrate to susceptible cell to activate the unique life cycle [18]. The larger pathogen’s form - initial, or reticular, body often observed in intracellular inclusions, rarer in exudates in a free state.
Complex and unique life cycle of Chlamydia trachomatis according to D. Taylor-Robinson [20] is shown in Figure 1.
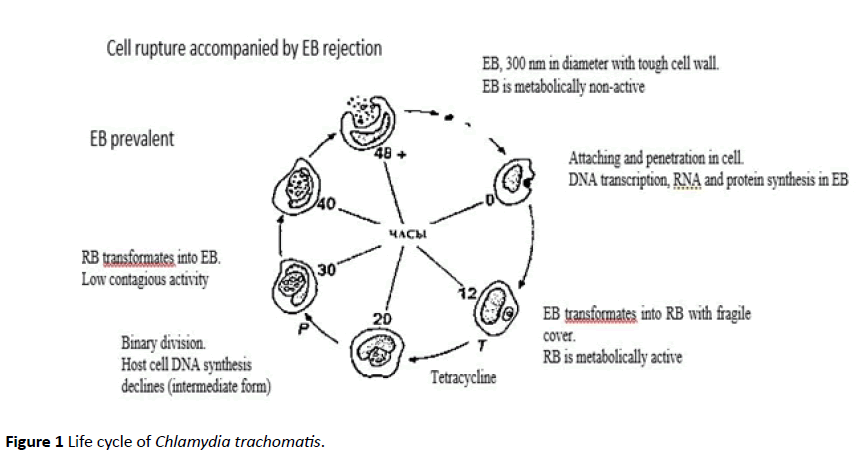
Figure 1: Life cycle of Chlamydia trachomatis.
In reproductive process asynchronous development of C. trachomatis is observed: under microscopic examination one can observe inclusions of each stage. That’s why antibacterial therapy courses should be prolonged including several medical groups. Unfortunately, it’s not always effective. Chronic C. trachomatis infection influences clinical manifestations in RA. We revealed a number of specific clinical features during our observation indicating on concomitant chronic C. trachomatis infection in patients with RA. According to our data about 15% of RA patients have co-existent C. trachomatis infection.
Thus, C. trachomatis infection in RA patients causes persistent high laboratory activity: increased ESR level, significant high RP and, therefore, SAA levels. Circumstantial evidence of persistent C. trachomatis infection in RA patients are join swelling with skin hyperemia at the disease onset, asymmetrical involvement of hand joints, arthritis of elbow joints on early disease stages or elbow joints contractures, involvement of (exceptions Rjoints, presence of enthestis, talalgia, longstanding low grade fever as well as hyperthermia accompanying methotrexate intake, high laboratory activity (ESR more than 50 mm/h) when low clinical signs of RA, seronegative RA with destruction of several joints, especially Vth metatarsophalangeal or interphalangeal foot joints. At the same time clinical manifestations of urinary tract involvement are usually poor.
All listed clinical manifestations may probable association of C. trachomatis infection and RA. However, in order to obtain precise results, one should use at least two diagnostic tools (e.g. polymerize chain reaction (PCR) and immune-enzyme analysis (IEA) or cultural method and IEA).
Therefore, the objective of present study was to assess possible influence of chronic Chlamydia trachomatis infection on secondary amyloidosis development in patients with rheumatoid arthritis.
Patients and Methods
We observed 104 RA patients in Belarusian Rheumatology centre. (ARA) [21].
All patients had undergone following tests: Clarification of medical history, previous medical data including joint lesion on the disease onset and present status; duration and effectiveness of Disease-Modifying Antirheumatic Drugs (DMARDs) intake, clinical analyses, tests for C. trachomatis infection with obligate PCR or cultural method (using McCoy medium). We also performed renal biopsy, rectal mucous biopsy or gingival biopsy with subsequent morphological analyze in RA patients having proteinuria, suspicious to secondary amyloidosis.
All patients were divided into two groups:
1st group: AA-positive RA patients (n=45).
2nd group: AA-negative RA patients (n=59).
There were no significant differences in patient’s age and sex, RA activity (SDAI), radiographic stage and RF positivity/ negativity between two groups.
Case Report
A 30-year-old woman, suffered from pain and swelling of I metacarpophalangeal articulation on right hand, V proximal interphalangeal joint, deformation of left elbow joint, pain in radiocarpal joint, knees, morning stiffness within an hour; foot edema.
Anamnesis morbi. In 1997 she developed right knee swelling. Patient referred to general practitioner, and five years received non-steriod anti-inflammatory drug and physiotherapeutic procedures. 1999 she was performed arthroscopy and partial synovectomy. In 2001 C. trachomatis infection was diagnosed by PCR-method; patient underwent 5 courses of antibiotics (without effect). During these five years she developed left knee and elbow as well as symmetric V proximal interphalangeal hand joint swelling. In 2006 patient developed morning joint stiffness, pain and symmetric swelling of radiocarpal joints; on radiographic data - joint space narrowing and erosions. She was made a diagnosis: rheumatoid arthritis associated with C. trachomatis infection. She was prescribed sulfasalazine 2 g/day. Two months later she discontinued treating because of adverse effect (gastrointestinal disorder). In august 2006 she was prescribed leflunomide 20 mg/day. In spring 2007 proteinuria in urine analysis was revealed (0.62 g/L), anemia (hemoglobin level 90 g/L) and increased ESR (73 mm/h) were revealed. In June 2007 AA-amyloidosis was histologically confirmed by nephrobiopsy. Renal function progressively declined (urea 23 mM/L, creatinine 450 μM/L, GFR 23 mL/min; proteinuria 3 g/L). On September 2008 patient was put on renal replacement therapy (hemodialysis).
Only 7 (11.9%) patients in the 2nd group presented asymmetric arthritis on the disease onset. None of them had skin hyperemia or longstanding hyperthermia or involved joints «exceptions R. All patients regularly received DMARDs, however low RA activity or disease remission were reached not in all cases.
As far as SAA detection in Belarusian clinics is not available, we assessed clinical disease activity as well as ESR and CRB levels. There is considered to be direct correlation between SAA and ESR (or CRP). In the 1st group ESR level was significantly higher than in the 2nd group: 55 (40; 67) mm/h in comparison with 36 (24; 52) mm/h .
Thus, longstanding C. trachomatis infection impacts clinical manifestations, outcome and effectiveness of basic DMARDs RA therapy, causes persistent high laboratory activity (ESR, and, hence, is a risk factor of secondary amyloidosis.
C. trachomatis infection in RA patients significantly correlates with the high probability of secondary amyloidosis development (R=0.93) ( <0.0001). In addition, odds ratio (OR) for C. trachomatis infection was 26.6; 95%CI 9.26-76.37. It means that in presence of Chlamydia trachomatis risk of secondary amyloidosis development increases in 26 times.
Based on the results of the analysis, the genotype SAA α/α (polymorphic loci 2995C/T and 3010C/T of the SAA1 gene) is a genetic risk factor for the development of amyloidosis as a complication of rheumatoid arthritis among Belarusian patients with RA (OR=45.26, CI 9,9062-206,8153). According to our analysis, the presence of C. trachomatis infection in combination with the SAA α/α genotype increases the risk of developing secondary amyloidosis in RA 55 times (OR=55) [22].
Results and Discussion
During medical history analysis and data of objective patient’s examination special attention was paid to clinical signs of C. trachomatis infection. 28 (62.2%) patients in 1st group presented an unusual onset of RA: knee swelling and synovitis, achillitis, asymmetric joint involvement (for example, right or left knee joint). At different stages of RA in 9 (20%) patients of 1st group joints swelling with skin hyperemia was observed. 2 (4.4%) AA-positive RA had atypical joint involvement on disease onset-I metacarpophalangeal articulation and V proximal interphalangeal joint. 8 (17.8%) patients of 1st group presented longstanding hyperthermia during disease course that can be a sign of chronic C. trachomatis infection. Notable, that 3 (6.7%) patients receiving DMARDs had hyperthermal reaction. Thus, antibacterial therapy normalized temperature. Almost all patients of 1st group had persistent high disease activity. Probably, it’s connected with irregular DMARDs receiving, which mentioned the majority of AA-positive patients. Besides, latent . trachomatis infection supported persistent RA activity according laboratory tests.
According our observations RA patients with increased risk of secondary amyloidosis characterized by number of clinical features. They have more often knee joints involvement on the disease onset (Â =0.03) and rarer-hand joints (Â =0.02). At the same time joint lesion is often asymmetric (Â =0.002). The DMARDs effectiveness usually is not enough. Moreover, in this patient’s group additional glucocorticoid drugs were necessary to achieve low clinical RA activity. Conversely in RA patients without secondary amyloidosis good effectiveness of standard DMARDs therapy (methotrexate or sulfasalazine) was observed.
We found out, that 38 (84.4%) of 45 AA-positive RA patients had concomitant C. trachÃmatis infection during all course of RA, diagnosed by PCR or cultural method using McCoy medium. In the 2nd group association of RA and C. trachomatis infection was revealed only in 10 (16.9%) of 59 patients (P=0.004).
Here we present a short case report describing secondary amyloidosis in RA patient associated with Ã. trachomatis infection.
Conclusion
Therefore, the presence of persistent C. trachomatis infection in patients with rheumatoid arthritis may act as a supplementary stimulus for the inflammation and, consequently, for increased SAA level. Thus, presence of C. trachomatis infection in RA patients is one of risk factors of renal amyloidosis. We consider that C. trachomatis can induce rapid progression of amyloid deposits and activate progression of “silent” amyloidosis. Clinical signs of C. trachomatis infection in RA patients (asymmetric arthritis, involvement of «exceptions R joints, longstanding hyperthermia) demand instant PCR-verification. Probably, course of antibiotics would allow to reduce risk of secondary amyloidosis and hence to improve life-span in patients with R. To estimate this hypothesis, further clinical studies should be conducted. All RA patients with C. trachomatis infection require dynamic renal function control conflicts of interests.
22814
References
- Anderson CJ, Gregory MC, Groggel GC, Clegg DO (1989) Amyloidosis and Reiter's syndrome: Report of a case and review of the literature. Am J Kidney Dis 14: 319- 323.
- Misra R, Wakhlu A, Krishnani N, Hissaria P, Aggarwal A (2004) Prevalence of silent amyloidosis in rheumatoid arthritis and its clinical significance. J Rheumatol 31: 1013-1014.
- Bergesio F, Ciciani AM, Santostefano M (2007) Renal involvement in systemic amyloidosis: An italian retrospective study on epidemiological and clinical data at diagnosis. Nephrol Dial Transplant 22: 1608-1618.
- Okuda Y, Takasugi K (1998) Diagnostic and prognostic study of secondary amyloidosis complicating rheumatoid arthritis. Amyloid and amyloidosis, Parthenon Publishing Group, New York.
- Soroka NF, Tushina AK, Danilenko NG, Sivitskaya LN (2011) Risk factors for developing renal amyloidosis in patients with rheumatoid arthritis. IJBM 1: 87-96.
- Carter JD, Gérard HC, Espinoza LR, Ricca LR, Marcet JV, et al. (2009) Chlamydiae as etiologic agent for chronic undifferentiated spondyloarthritis. Arthritis Rheum. 60: 1311-1316.
- Uhlar CM, Whitehead AS (1999) The kinetics and magnitude of the synergistic activation of the serum amyloid A promoter by IL-1 beta and IL-6 is determined by the order of cytokine addition. Scand J Immunol 49: 399-404.
- Betts JC (1993) The Role of NF and NF-IL6 transactivating factors in the synergistic activation of human serum amyloid A gene expression by interleukin-1 and interleukin-6. J Biol Chem 268: 25624-25631.
- Migita K, Abiru S, Nakamura M, Komori A, Yoshida Y, et al. (2004) Lipopolysaccharide signalling induces serum amyloid A(SAA) synthesis in human hepatocytes in vitro. FEBS Lett 569: 235-239.
- Hutchinson WL, Noble GE, Hawkins PN, Pepys MB (1994) The pentraxins, C-reactive protein and serum Amyloid P component, are cleared and catabolized by hepatocyes in vivo. J Clin Invest 94: 1390-1396.
- Kisilevsky R (2000) The relation of proteoglycans, serum Amyloid P and apo E to amyloidosis current status. Amyloid 7: 23-25.
- Cunnane G, Grehan S, Geoghegan S, Mc Cormack C, Shields D, et al. (2000) Serum amyloid A in the assessment of early inflammatory arthritis. J Rheumatol 27: 58-63.
- Urieli-Shoval S, Linke RP, Matzner Y (2000) Expression and function of serum amyloid A, a major acute-phase protein, in normal and disease states. Curr Opin Hematol 7: 64-69.
- Bakkaloglu A, Duzova A, Ozen S, Balci B, Besbas N, et al. (2004) Influence of Serum amyloid A (SAA1) and SAA2 gene polymorphisms on renal amyloidosis, and on SAA/C-reactive protein values in patients with familial mediterranean fever in the Turkish population. J Rheumatol 31: 1139–1142.
- Van Gameren II, Vellenga E, Hazenberg BPC (2009) Novel treatment for systemic amyloidosis. J Clin Rheumatol 4: 171-188.
- Lachmann HJ, Goodman HJ, Gilbertson JA, Gallimore JR, Sabin CA, et al. (2007) Natural history and outcome in systemic amyloidosis. N Engl J Med 356: 2361-2371.
- Gillmore JD, Lovat LB, Persey MR, Pepys MB, Hawkins PN (2001) Amyloid load and clinical outcome in AA amyloidosis in relation to circulating concentration of serum amyloid A protein. Lancet 358: 24- 29.
- Bavoil PM, Hsia R, Ojcius DM (2000) Closing in on Chlamydia ant its intracellular bag of tricks. Microbiology 146: 2723-2731.
- Brunham RC, Rey-Ladino J (2005) Immunology of chlamydia infection: Implication for a Chlamydia vaccine. Nat Rev Immunol 5: 149-161.
- Taylor-Robinson D, Keat A (2001) How can a causal role for small bacteria in chronic inflammatory arthritis’s be established or refuted? Ann Rheum Dis 60: 177- 184.
- Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31: 315-324.
- Soroka NF, Tushina AK (2014) Secondary amyloidosis in Belarusian patients with rheumatoid arthritis (1stedn), Lambert Academic Publishing, New Jersey, USA.






