Keywords
Brain atrophy; Chronic subdural hematoma; Low density; Neurological comorbidities; Surgical drainage
Abbreviations
CG: Conservative Group; CSDH: Chronic Subdural Hematoma; CT: Computed Tomography; Ml: Milliliter; MMSE: Mini-Mental State Examination; mRS: Modified Rankin Scale; MRI: Magnetic Resonance Imaging; SG: Surgical Group
Introduction
Chronic subdural hematoma (CSDH) is a predominantly neurological condition usually affecting elderly individuals, and resulting from trauma, coagulopathy, anticoagulant therapy and vascular malformation [1-3] Although CSDH is a serious and frequent entity, there is no consensus regarding the optimal treatment strategy. Response to surgery has been so satisfactory that this is generally considered the treatment of choice. However, large studies have shown that older age independently contributes to increase the rate of lethality, complications and recurrences after the surgical drainage of chronic SDH [3-5].
The role of hematoma density in CSDH recurrence has been studied previously, but the results have not been consistent [6,7]. Compared with high- or mixed-density of CSDH, hypodense hematoma is not an acute condition and it is rational to recommend for close observation. Due to significant risk and poor outcome associated with surgery, currently some authors had an attempt to treat this subtype of CSDH with conservative method, and achieved good outcome with lower rates of lethality and recurrence [8,9]. These evolving results present challenges for the surgeon in deciding whether to surgically drain a hypodense CSDH or to manage them conservatively in elderly patients. In this paper, we performed a retrospective study to compare the neurologic outcomes between surgical drainage and conservative management of CSDH with hypodense on initial CT scan in patients aged 70 years or older.
Materials and Methods
Patient’s population
We identified patients by retrospective analysis of the medical records and neuroradiographic studies for all patients in our hospital from September 2008 to July 2015, in whom CSDH was diagnosed by initial computed tomography (CT) scans. In addition, magnetic resonance imaging (MRI) was essentially performed when subdural hygroma were originally suspected. Only high signal presenting in both T1- and T2-wighted image was diagnosed as a CSDH. Only patients who were ≥ 70 years old, and who had a low-density of CSDH on CT scan were enrolled in this study. Forty-one patients suffered from unilateral CSDH, and twelve patients showed bilateral CSDH. Patients were classified according to treatment into a surgical group (SG) and a conservative group (CG), based on the patient's physical exam, neurologic exam, medical status, image findings, and the wishes of the patient and/or their family.
Data acquisition and comparison between the two groups was performed by analyzing the patients’ demographics (sex, age), main symptoms, medical histories, mini-mental state examination (MMSE), and modified Rankin Scale (mRS) score. Radiological examinations and imaging-related parameters of the CSDH were also were recorded and used to check whether the 2 patient groups were similar in these variables. The neurological co-morbidities of patients were established in the presence of at least one of the following criteria: 1) a documented history of ischemic or hemorrhagic stroke; 2) infarcts on the CT scan and/ or MRI performed on admission; and 3) a documented history of dementia, Parkinson diseases and/or hydrocephalus at least six months [10,11].
Clinical and imaging evaluations
Neurological status of patients was evaluated at the time of diagnosis, and in the second weeks, and 1-month, 6-month, and 12-month after treatment. Favorable and unfavorable outcomes were defined as mRS scores of 0-3 and 4-5, respectively [12]. Any neurological improvement in the surgical and conservative groups was recorded. This improvement was defined as a decrease by at least 1 score in the neurological status of patients based on the mRS scores. Patient cognitive ability was also assessed compared to their mental status pre- and post-operation as determined by MMSE score.
According to the initial CT scans, brain atrophy was divided into three stages: none or mild atrophy, moderate atrophy, such as dilated sulci and sylvian fissure, and severe atrophy, such as widely dilated sulci and subdural space [7,13]. Presence of midline shift, brain atrophy and hematoma location (right, left, bilateral) was determined on CT scans obtained immediately before therapy intervention. The cut-off values for the degree of midline shift were defined based on previous reports [14]. The volume (in milliliters) of the hematoma was calculated on the basis of pre- and post-procedural CT films at the different followup time points.
Statistical analysis
Data analyses were performed using the SPSS for Microsoft Windows (Version 13.0; SPSS, Inc., Chicago, IL). The Pearson’s chi-square test or Fisher exact test was used to compare discontinuous variables to determine statistical significance of any differences. For continuous measures Student t-tests and Wilcoxon’s z-test/Kruskal-Wallis test were used. To compare the volumes of hematoma before and after treatment, independent t-tests were used. P values of less than 0.05 were considered statistically significant. In addition, the logistic regression was performed to assess the impact of the measured variables on the recurrence/enlargement of CSDH after treatment.
Results
Patient’s demographics
The demographic and clinical data of all patients are summarized in (Table 1). The mean age of patients was 80.9 ± 7.1 years in the surgical group and 82.5 ± 6.9 years in the conservative group. Twenty-two patients (41.5%) had one or more significant neurological co-morbidities on admission, including stroke, dementia, Parkinson disease or hydrocephalus. Generally, the groups did not differ significantly with respect to demographic features.
| Variables |
Surgical group |
Conservative group |
Total
(n=53) |
P Value |
| Sex |
| M/F |
23/8 |
17/5 |
40/13 |
0.797 |
| Age (years) |
80.9 ± 7.1 (70-93) |
82.5 ± 6.9 (70-94) |
81.5 ± 6.9 |
0.437 |
| Pathogenesis |
| Head injury |
21 (67.7) |
14 (63.6) |
35 (66) |
0.638 |
| Antiplatelet therapy |
7 (22.6) |
7 (31.8) |
14 (26.4) |
- |
| Unknown |
3 (9.7) |
1 (4.6) |
4 (7.6) |
- |
| Main symptoms† |
| Headache |
12 (38.7) |
7 (31.8) |
19 (35.8) |
0.606 |
| Dizziness |
20 (64.5) |
11 (50) |
31 (58.5) |
0.291 |
| Cognitive decline |
14 (45.2) |
11 (50) |
25 (47.2) |
0.728 |
| Urinary incontinence |
5 (16.1) |
5 (22.7) |
10 (18.9) |
0.545 |
| Drowsiness |
3 (9.7) |
3 (13.6) |
6 (11.3) |
0.683 |
| Aphasia |
4 (12.9) |
3 (13.6) |
7 (13.2) |
1.0 |
| Paralysis |
17 (54.8) |
10 (45.5) |
27 (50.9) |
0.501 |
| Background disease |
| Hypertension |
16 (51.6) |
13 (59.1) |
29 (54.7) |
0.590 |
| Diabetes |
8 (25.8) |
6 (27.3) |
14 (26.4) |
0.905 |
| Heart disease |
11 (35.5) |
10 (45.5) |
21 (39.6) |
0.465 |
| Stroke |
8 (25.8) |
7 (31.8) |
15 (28.3) |
0.632 |
| Parkinson |
3 (9.7) |
1 (4.6) |
4 (7.6) |
0.633 |
| Hydrocephalus |
5 (16.1) |
2 (9.1) |
7 (13.2) |
0.686 |
| Dementia |
10 (32.3) |
6 (27.3) |
16 (30.2) |
0.697 |
| Radiological data |
| Location of hematoma |
| Unilateral |
23 (74.2) |
18 (82.6) |
41 (78.4) |
0.513 |
| Bilateral |
8 (25.8) |
4 (17.4) |
12 (21.6) |
|
| Brain atrophy |
| No or mild |
2 (6.5) |
2 (9.1) |
4 (7.5) |
0.830 |
| Moderate |
18 (58.1) |
11 (50) |
29 (54.8) |
|
| Severe |
11 (35.5) |
9 (40.9) |
20 (37.7) |
|
| Shift of middle line |
| <10mm |
17 (54.8) |
16 (72.7) |
33 (62.3) |
0.186 |
| =10mm |
14 (45.2) |
6 (28.3) |
20 (37.7) |
|
| Patients improved (mRS) |
| 1 months |
26 (83.8) |
12 (54.5) |
38 (71.7) |
0.02 |
| 12 months |
27 (87.1) |
16 (72.7) |
43 (81.1) |
0.287 |
| Death |
1 (3.2) |
2 (9.1) |
3 (5.6) |
0.563 |
The Pearson’s chi-square test or Fisher exact test.
* Values represent the mean ± SD (range), unless indicated otherwise.
† Some patients had more than one symptom.
No: Number; CSDH: Chronic Subdural Hematoma; SD: Standard
Deviation; M/F: Male/Female; mRS: modified Rankin Scale.
Table 1: Clinical characteristics and findings of the patients with CSDH in this study*.
As expected, despite a similar average initial volume, patients undergoing surgical drainage had significantly greater average volume reduction and lower final volumes than those in the conservative group. The differences between the two groups were firstly observed at the 30-day follow up, with residual volume of 32.3ml and 54.8ml in the SG and CG (P=0.003), respectively. Since then, the residual of hematoma reduced sharply in both groups, whereas the difference in volume between the two groups remained significantly, but began to narrow, and disappeared until the latest follow up (Figure 1).
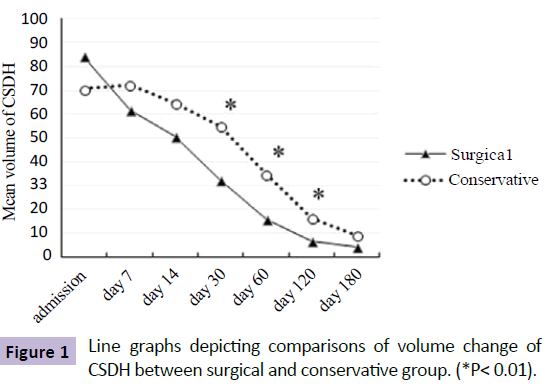
Figure 1: Line graphs depicting comparisons of volume change of CSDH between surgical and conservative group. (*P< 0.01).
Clinical outcome
Overall, the neurological and mental status of patients in both group were significant improvement from admission to followup (Table 2). However, nearly 84% of patients were significantly improved in neurological status at least 1 point on 1-month follow-up examination in the SG (26/31), whereas only 55% (12/22) of patients achieved a favourable outcome in the CG (P=0.02), yet no statistically significant difference on mean mRS scores was observed between the two groups.
| Factors |
Surgical group |
Conservative group |
P Value |
| mRS |
| Day 0 |
3.29 ± 1.22 (1-5) |
3.36 ± 1.29 (1-5) |
0.874 |
| Day 14 |
2.77 ± 1.36 (1-5)* |
3.36 ± 1.25 (1-5) |
0.111 |
| 1 month |
2.39 ± 1.31 (0-5)* |
2.91 ± 1.48 (0-5)* |
0.149 |
| 6 months |
2.03 ± 1.58 (0-5)* |
2.41 ± 2.01 (0-6)* |
0.557 |
| 12 months |
2.06 ± 1.65 (0-6)* |
2.41 ± 2.02 (0-6)* |
0.582 |
| MMSE† |
| Day 0 |
20.45 ± 4.37 (13-27) |
20.05 ± 4.73 (14-29) |
0.520 |
| Day 14 |
20.87 ± 4.14 (13-28) |
19.75 ± 4.23 (12-29) |
0.352 |
| 1 month |
22.48 ± 4.02 (15-28)* |
20.73 ± 5.10 (14-29) |
0.174 |
| 6 months |
23.29 ± 4.25 (14-29)* |
22.71 ± 4.31 (15-29)* |
0.656 |
| 12 months |
23.60 ± 3.96 (16-29)* |
22.70 ± 4.29 (14-29)* |
0.450 |
†The values of MMSE were rejected in the statistical analysis for patients who died during follow-up.
Compared with the values on admission, *P<0.01; MMSE: Mini-Mental State Examination; SD: Standard Deviation; mRS: modified Rankin Scale.
Table 2: Neurological and mental status from admission to follow up in patients between surgical and conservative groups (Mean ± SD).
Of note, patients aged 80 or older presented with worse neurological status from admission to follow up compared to younger patients, irrespective of whether these hemorrhages are drained or not. For patients aged 80 or older, there was significant improvement in neurological status from admission to 1-month follow-up in the SG (admission mRS: 3.65; followup mRS: 2.75; P=0.0001), while no significant change was seen in the CG (admission mRS: 3.80; follow-up mRS: 3.47; P=0.096) (Figure 2). On the contrary, there was a significant improvement in neurological outcome for patients younger than 80 years in both groups after treatment (Figure 3).
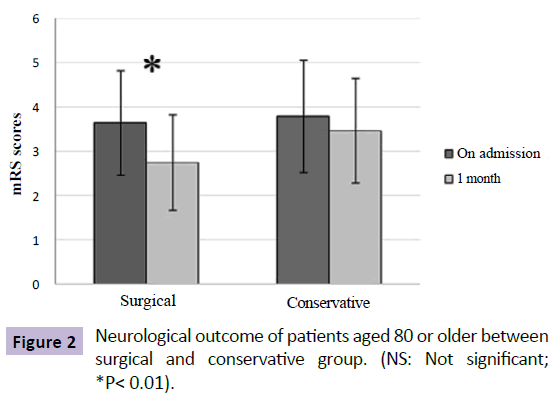
Figure 2: Neurological outcome of patients aged 80 or older between surgical and conservative group. (NS: Not significant; *P< 0.01).
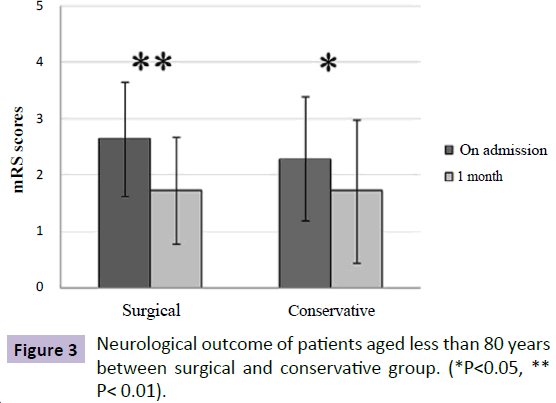
Figure 3: Neurological outcome of patients aged less than 80 years between surgical and conservative group. (*P<0.05, ** P< 0.01).
For patients presenting with an mRS score of 1-3 on admission (SG mRS score: 2.47; CG mRS score: 2.33; P=0.483), the improvement in neurological status of patients undergoing surgical drainage compared to patients with conservative alone was apparent as early as 2 weeks after treatment (SG mRS score: 1.84; CG mRS score: 2.42; P=0.041) (Figure 4). Similarly, patients presenting with an admission mRS score of 4-5 in the SG tended to have a better neurological status on 1-month follow up exam than patients in the CG (SG mRS score: 3.67; CG mRS score: 4.1; P=0.13), despite lack of statistical significance between the two groups from admission to follow up.
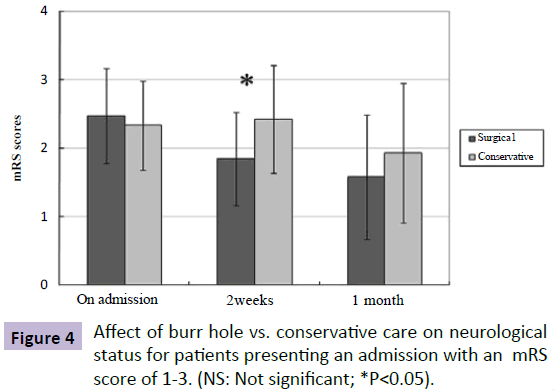
Figure 4: Affect of burr hole vs. conservative care on neurological status for patients presenting an admission with an mRS score of 1-3. (NS: Not significant; *P<0.05).
Interestingly, patients presenting with an admission mRS score of 4-5 in the SG had a higher scores in their mental status on 1-month follow-up compared to those in the CG as determined by MMSE (MMSE score in SG: 19.3; MMSE score in CG: 16.3, P=0.031) (Figure 5). However, no significant difference in mental status was observed in patients presenting with an admission mRS score of 1-3 at different time points between the SG and the CG.
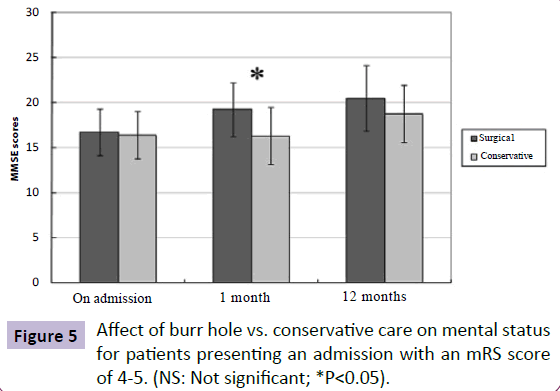
Figure 5: Affect of burr hole vs. conservative care on mental status for patients presenting an admission with an mRS score of 4-5. (NS: Not significant; *P<0.05).
Affect of brain atrophy on neurological outcome
According to Neal's method of determining the brain atrophy in post-procedural CT scans, all of our patients had atrophy in different degree. For patients with severe brain atrophy, the initial mRS scores on admission did not significantly differ between the SG and the CG (SG mRS score: 3.85; CG mRS score: 4.22; P=0.351). After treatment, the mean mRS of patients on 1-month follow up in the SG and the CG were 2.92 and 4.0, respectively, demonstrating statistical significance between the two groups (P=0.036), and this difference remained significantly on 1-year follow-up exam (SG mRS score: 2.58;CG mRS score: 4.11; P=0.029) (Figure 6).
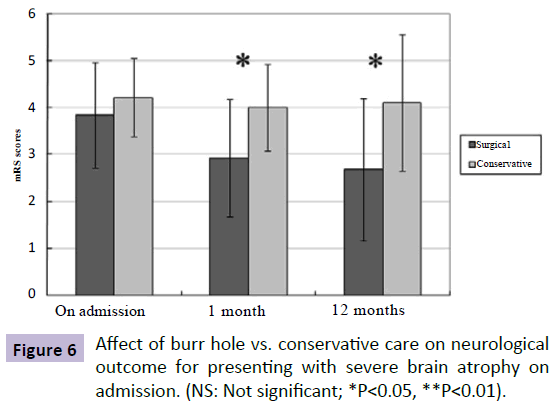
Figure 6: Affect of burr hole vs. conservative care on neurological outcome for presenting with severe brain atrophy on admission. (NS: Not significant; *P<0.05, **P<0.01).
In this subgroup, patients undergoing surgical drainage tended to have a better mental status on admission than patients that received conservative care (SG MMSE score: 18.7; CG MMSE score: 16.4, P=0.132). Furthermore, surgical patients showed greater improvement in mental outcome on 1-month follow-up compared to conservative patients (SG MMSE score: 20.6; CG MMSE score: 16.6, P=0.011). At 1-year follow-up exam, however, there was also no significant difference in the mental status in patients between the two groups (SG MMSE score: 21.9; CG MMSE score: 18.7, P=0.082) (Figure 7).
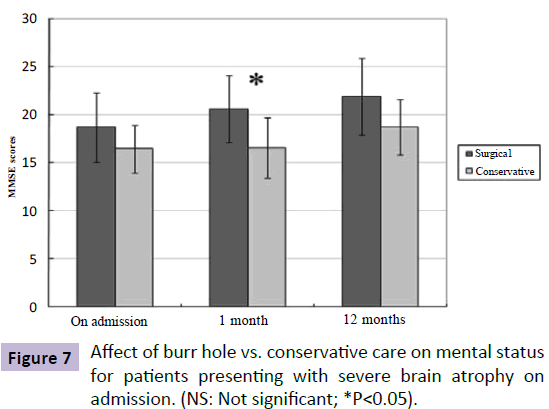
Figure 7: Affect of burr hole vs. conservative care on mental status for patients presenting with severe brain atrophy on admission. (NS: Not significant; *P<0.05).
In contrast there was no significant difference in either mRS or MMSE scores in patients that initially presented with a mild or moderate atrophy at different time points between the SG and the CG (data not shown).
Affect of neurological co-morbidities on neurological outcome
To assess the result of the different treatment in patient with neurological co-morbidities, we compared the neurological status before and after treatment in patients with preexisting co-morbidities between the SG and the CG. After treatment, there was more patients showing good neurological recovery to their baseline status on the latest follow-up exam in the SG than those in the CG (76.9% vs 33.3%, P=0.031), despite lack of statistical significance in mean mRS scores on 1-month follow-up between the two groups (SG mRS score: 3.3; CG mRS score: 3.8; P=0.218). However, there was statistically significant difference in neurological status on 1-year follow-up exam between the two groups (SG mRS score: 3.08; CG mRS score: 4.33; P=0.026) (Figure 8).
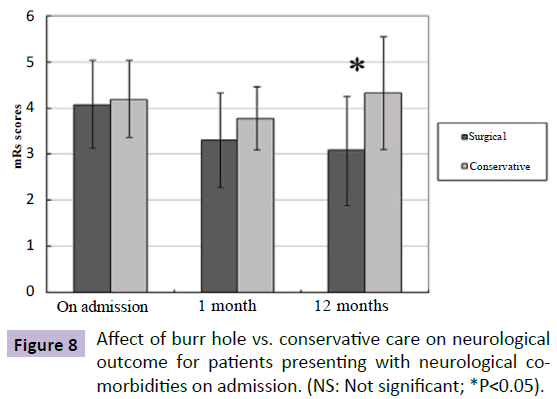
Figure 8: Affect of burr hole vs. conservative care on neurological outcome for patients presenting with neurological comorbidities on admission. (NS: Not significant; *P<0.05).
Similarly, there was significant improvement in mental status from admission to follow-up in the SG (admission MMSE: 17; 1-month follow-up MMSE: 19.5; P=0.001), while no significant mental change was seen in the CG (admission MMSE: 17.1; follow-up MMSE: 18.1; P=0.253). Unsurprisingly, there was certainly no statistically significant difference in MMSE scores in patients with neurological co-morbidities at different time points between the SG and the CG (Figure 9).
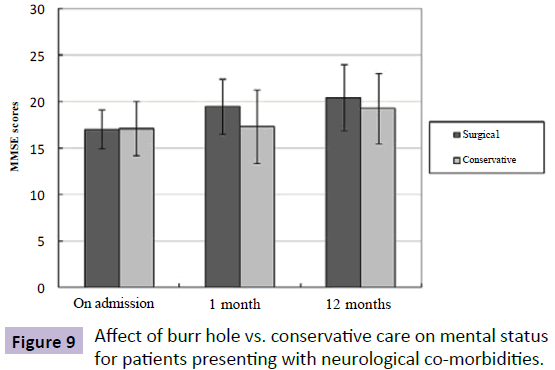
Figure 9: Affect of burr hole vs. conservative care on mental status for patients presenting with neurological co-morbidities.
For patients who had no definitely neurological co-morbidities, there was significant improvement in neurological status from admission to 1-month follow-up in the SG (admission mRS: 2.72; follow-up mRS: 1.72; P=0.001), while no significant change was seen in the CG (admission mRS: 2.69; follow-up mRS: 2.31; P=0.09). However, the mental status of patients in both groups was significant improvement from admission to 1-month follow-up, though no statistically significant difference in mean MMSE score was observed between the SG and the CG (followup MMSE in SG: 24.6; follow-up MMSE in CG: 23.1; P=0.374) (data not shown).
Complications and recurrence/enlargement of hematomas
In total, 3 patients died during follow-up, 2 from the conservative group and one from the surgical group, due to general complications resulting from multiple medical co-morbidities within 1-year after treatment. Significant postoperative complications directly related to surgery was found in three cases (9.6%), including acute SDH, postoperative intra-parenchymal bleeding and seizures, which were managed conservatively with favorable outcome. However, patients in the CG seemed particularly prone to systemic complications (e.g. pneumonia and urinary tract infection) during their inpatient stay, though there was no significant difference between the two groups (SG: 16.1%; CG: 31.8%; P=0.179).
Only one patient in the SG experienced recurrence 3 months after first surgery, who received repeated drainage and subsequently achieved satisfactory resolution of his SDH. Four patients (18.2%) in the CG were subjected to relapsing of their dementia and hemiparesis from one to six months after discharge, and subsequently confirmed enlargement of the residual hematomas on the emergency CT scan. Three of these 4 patients then underwent burr-hole surgery and experienced clinical improvement (Figure 10). The remaining one patient aged 85 years abandoned surgical intervention due to their poor physical status, and died four months later. Logistic regression indicated that severe brain atrophy was significantly associated with recurrence or enlargement of the chronic SDH (P=0.001; 95% CI=0.048-0.333).
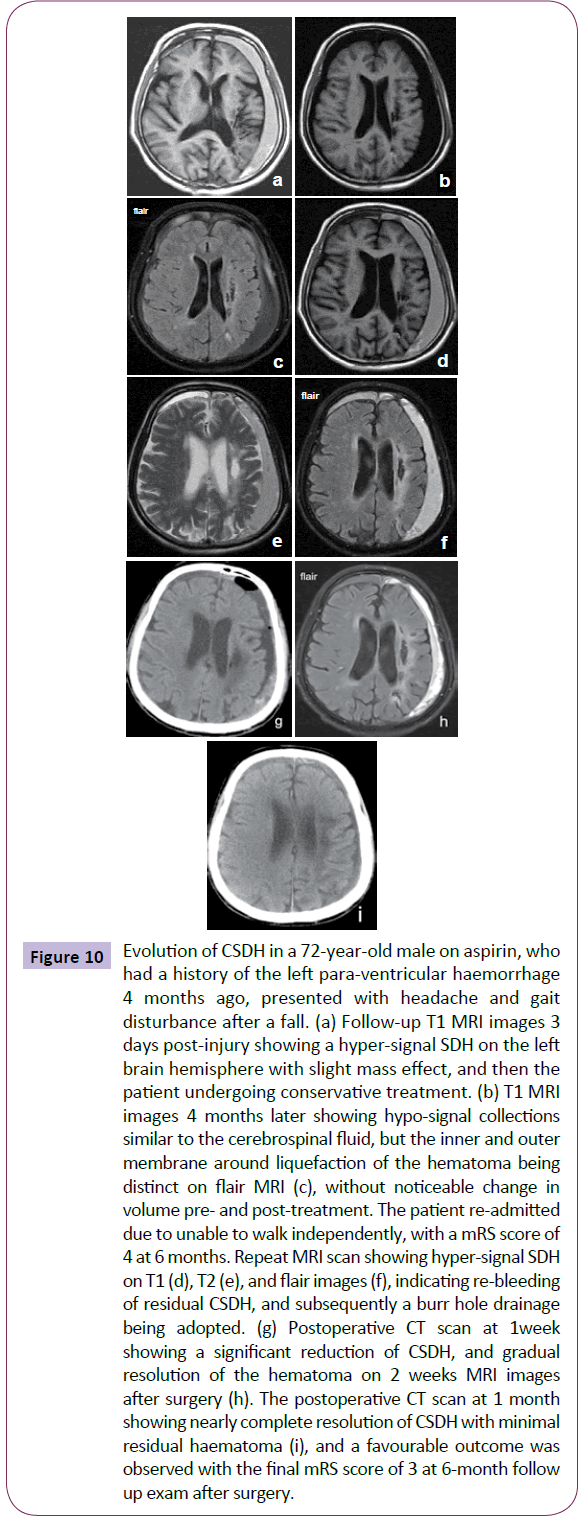
Figure 10: Evolution of CSDH in a 72-year-old male on aspirin, who had a history of the left para-ventricular haemorrhage 4 months ago, presented with headache and gait disturbance after a fall. (a) Follow-up T1 MRI images 3 days post-injury showing a hyper-signal SDH on the left brain hemisphere with slight mass effect, and then the patient undergoing conservative treatment. (b) T1 MRI images 4 months later showing hypo-signal collections similar to the cerebrospinal fluid, but the inner and outer membrane around liquefaction of the hematoma being distinct on flair MRI (c), without noticeable change in volume pre- and post-treatment. The patient re-admitted due to unable to walk independently, with a mRS score of 4 at 6 months. Repeat MRI scan showing hyper-signal SDH on T1 (d), T2 (e), and flair images (f), indicating re-bleeding of residual CSDH, and subsequently a burr hole drainage being adopted. (g) Postoperative CT scan at 1week showing a significant reduction of CSDH, and gradual resolution of the hematoma on 2 weeks MRI images after surgery (h). The postoperative CT scan at 1 month showing nearly complete resolution of CSDH with minimal residual haematoma (i), and a favourable outcome was observed with the final mRS score of 3 at 6-month follow up exam after surgery.
Discussion
CSDH is a condition mostly present in elder people, and the majority of patients had a history of head trauma, as noted in our series and in the literature [1,2]. The term “chronic” subdural hematoma has been loosely defined, with various authors applying it to those in the different stage of CSDH. According to CT scan, hematoma density is known to decrease with time, passing from a high-stage to an iso-and finally a low density stage [15,16]. Therefore, lower hematoma densities are expected to be seen in the late stage of hematoma course. In concern to the clinical outcome, it is not clear which type of hematoma is suited for surgical drainage for each patient. Some advocated that the operating in the homogeneous stage of CSDH, may reduce the recurrence rate [6,17]. Others persisted that low density hematoma often lead to poor outcome after surgery, compared to other type of CSDH [7,18]. Furthermore, the long-term outcome of elderly patients and the role of conservative management versus surgery are still contradictory based on the published data [7,8,11,18]. In this study, we evaluate the neurologic outcome of patients who underwent burr hole drainage or conservative alone for their CSDH with low density on initial CT scan.
Overall, the neurological and mental status of patients was significant improvement after surgical drainage of a CSDH. The difference between two treatment groups was proclaimed at the 30-day follow-up, with a rate of favorable outcome of 84% and 55% in the surgical and conservative care groups, respectively. Parlato et al. reported on a series of a 24 cases of CSDH and concluded that patients with age over 70 years, worsening mental function, brain atrophy, and absence of increase of intracranial pressure are indication for conservative therapy. However, the author demonstrated that surgery should be performed immediately for patients if the symptoms have not relieved after 7 or 10 days of clinical observation [19]. Importantly, symptoms relief of patients did not translate into functional improvements after conservative treatment, while surgical drainage of CSDH may results in rapid improvement of patient’s status in a shortterm period, avoiding the aggravation of diseases.
Previous studies have shown that older age, concomitant disease and poor admission neurological status are related to worse outcomes and increased mortality in patients undergoing surgical treatment for CSDH [20,21]. Given the steady increase in the elderly population, diseases associated with old age will become more common, which may lead to an overall decline from the neurological baseline before CSDH onset in this population. Many neurosurgeons advocated that the operative risks in patients over 80 years are prone to outweigh the benefits of surgery due to poor baseline function [3,19,21,22]. In our study, patients over 80 years had significant improved in neurological status on 1-month after surgery while no statistical difference in patients with conservative treatment. Recent study suggested that surgery for CSDH is safe and positively recommended even in patients over 90 years old, with a lower rate of 6-month death [16]. The findings are consistent with our results, suggesting that patients over 80 years may be similarly able to return to their baseline function compared with younger patients, but may require longer hospital stays for further postoperative rehabilitation.
Another significant factor determining outcome is the initial neurological status on admission. In our series, patients with an initial mRS score of 0-3 often achieved a favorable outcome, regardless of treatment manner, which was in accordance with previous reports [9,23,24]. For some incidental CSDH, however, the neurological status of patients often determined by both CSDH and other concomitant diseases, made it difficult to precisely evaluate their baseline function on admission. Over one-third of our patients (i.e., 28%, stroke; 13%, hydrocephalus; 30%, dementia) presented definitely compromised neurological conditions before CSDH onset. Such patients are susceptible to pneumonia and urinary tract infections, and then become progressively more bedbound and dependent if conservative treatment is adopted, which may eventually deteriorate their neurological function. However, surgical treatment can result in improvement of cognitive ability in a short-term (1-month follow-up), and improvement of neurological status in a longterm (1-year follow-up), despite some patients will remain dependent with limited mRS scores. This is in contrast to past research [25,26], but more recent studies have shown the similar results to our findings [15,16].
Whether the degree of brain atrophy affecting the surgical outcomes in patients with CSDH or not remains controversy. Considering the association of brain atrophy with age, patients age over 80 years have poor outcome due to preexisting comorbidities and brain atrophy [7,18,21]. Using multivariate logistic regression analysis, Amirjamshidi et al. [7] found that brain atrophy independently increases the risk of unfavorable outcome after drainage of CSDH. However, another study revealed that brain atrophy was not a significant predictor of successful treatment [13]. Furthermore, patients with brain atrophy are often lack of clinical and radiological signs of high intracranial pressure that allow one to choose conservative treatment [19]. Unfortunately, conservative alone was not always successful, a CSDH with observation will not completely disappear but rather than translate into a hygroma over time, especially in elder patients with atrophy, which is a potential risk factor of re-bleeding or enlargement of residual hematoma [9,22]. In our material, patients with severe atrophy initially presented with worse neurological function compared to patients with mild or moderate atrophy. Even so, the results of our study indicate that patients with severe atrophy may also benefit from surgical drainage of a chronic SDH, with a better outcome in either neurological function or mental status, compared to patients with conservative treatment. These findings are consistent with other reports [16], and brain atrophy is not a contraindication for surgical drainage.
The total rate of mortality of 3.1% after surgery was lower compared to those previously reported rates range from 8%-44% [2,3,14]. There was not different in respect of rate of mortality between two groups in our cohort, unlike previous reports [21,27]. Sun et al. reported that the mortality rates of the corticosteroid monotherapy group, the combination group and the surgery groups were 4%, 3%, 15%, respectively, and therefore suggested that steroid treatment in a selected group of patients is a good option, particularly in patients with co-morbidity. However, Lee et al. [16] emphasized that surgical drainage of a CSDH in patient ≥ 90 years is associated with a lower rate of inpatient death and higher 6-month survival rates than for patients who receive only conservative care. This suggests that elderly patients who have preexisting co-morbidities can also benefit from surgical drainage of a CSDH with lower risks of perioperative complications. With recent advances in surgical techniques and perioperative care, morbidity and mortality rates have generally decreased, and surgical management may be associated with more favorable outcomes in these patients.
The rate of recurrence for surgically drained CSDH was lower in our group. This may be attributed to sufficient irrigation from different directions (frontal, infratemporal, occipitalia) during operation, which establish a connection with all other hematoma compartments and provide a washout of blood products and other factors possibly involved in maintaining the hematoma [28,29]. Brain atrophy provides a potential space for hematoma expansion, which might be associated with a poor outcome. In our logistic regression analysis, we found that only severe brain atrophy was significantly associated with enlargement of CSDH. This result is in line with previous reports [27,28,30]. Some giant hematoma in patients with severe brain atrophy had taken more than one year to be fully resolved even after a successful operation. The resolution of a CSDH depends mainly on the extent of brain re-expansion, making it less likely that the CSDH resolves with conservative treatment alone in elderly patients with brain atrophy. Based on a longer clinical review of one year, however, our data indicate that surgical drainage of CSDH may be attribute to improving neurological status even in patients whose brain has atrophied, and ultimately leads to the resolution of residual hematoma in the majority of patients.
Limitation
The present study was a retrospective study and is therefore subject to potential sources of bias and variation. The number of the patients was small, and the hospital costs and inpatients stay was not analyzed between the two groups in the present study. Considering of the final neurological outcome, a need exists to better define the treatment option tailored for each patient. Therefore, further randomized, prospective clinical trials with large samples are required to refine the indications for the different treatment modalities of CSDH based on the patient care and outcomes, as well as hospital costs.
Conclusion
Our data suggest that surgical drainage for low density of CSDH is safe and can be positively recommended even for elderly patients with seriously brain atrophy and preexisting neurological co morbidities. The conservative treatment may be beneficial and cost-effective in the treatment of CSDH with a mRS ≤ 3. From long-term observation, however, it demonstrates that the nonsurgical treatment does not reduce the incidence of re-bleeding from residual CSDH compared to the surgical treatment due to poor brain expansion in the elderly. The complications are not associated with the operation, and often arise from other medical comorbidities. Despite a possible longer hospital stay, elderly patients frequently experience significant neurological improvement after surgery for their CSDH.
Funding
This work was partially supported by grants from shanghai's Program for Senior Chinese and Western Medicine Personnel Training Project (Nos. VH 01.26.013).
Conflicts of Interests
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Ethics
The research process was carried out following obtaining the informed consent forms which were in accordance with the ethical standards of the Hospital's Institutional Review Board on Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine.
9819
References
- Chen JC, Levy ML (2000) Causes, epidemiology, and risk factors of chronic subdural hematoma. Neurosurgery Clinics of North America 11:399-406.
- Almenawer SA, Farrokhyar F, Hong C, Alhazzani W, Manoranjan B, et al. (2014) Chronic subdural hematoma management: a systematic review and meta-analysis of 34829 patients. Ann Surg 259:449-457.
- Liu W, Bakker NA, Groen RJ (2014) Chronic subdural hematoma: a systematic review and meta-analysis of surgical procedures. J Neurosurg 121:665-673.
- Santarius T, Kirkpatrick PJ, Ganesan D, Chia HL, Jalloh I, et al. (2009) Use of drains versus no drains after burr-hole evacuation of chronic subdural hematoma: Arandomised controlled trial. Lancet 374: 1067-1073.
- Berghauser Pont LM, Dirven CM, Dippel DW, Verweij BH, Dammers R (2012) The role of corticosteroids in the management of chronic subdural hematoma: A systematic review. European Journal of Neurology 19: 1397-1403.
- Ohba S, Kinoshita Y, Nakagawa T, Murakami H (2013) The risk factors for recurrence of chronic subdural hematoma. Neurosurg Rev 36:145-150.
- Amirjamshidi A, Eftekhar B, Abouzari M, Rashidi A (2007) The relationship between Glasgow coma/outcome scores and abnormal CT scan findings in chronic subdural hematoma. ClinNeurolNeurosurg 109:152-157.
- Hirashima Y, Kurimoto M, Nagai S, Hori E, Origasa H, et al. (2005) Effect of platelet-activating factor receptor antagonist, etizolam, on resolution of chronic subdural hematoma -A prospective study to investigate use as conservative therapy. Neurol Med Chir (Tokyo) 45:621-626.
- Kageyama H, Toyooka T, Tsuzuki N, Oka K (2013) Nonsurgical treatment of chronic subdural hematoma with tranexamic acid. J Neurosurg 119:332-337.
- Spagnoli D, Innocenti L, Bello L, Pluderi M, Bacigaluppi S, et al. (2006) Impact of cerebrovascular disease on the surgical treatment of idiopathic normal pressure hydrocephalus. Neurosurgery 59:545-552.
- Kiefer M, Eymann R, Steudel WI (2006) Outcome predictors for normal-pressure hydrocephalus. ActaNeurochirSuppl 96:364-367
- Borger V, Vatter H, Oszvald Á, Marquardt G, Seifert V, et al. (2012) Chronic subdural hematoma in elderly patients: a retrospective analysis of 322 patients between the ages of 65-94 years. ActaNeurochir (Wien) 154:1549-1554.
- Neal MT, Hsu W, Urban JE, Angelo NM, Sweasey TA, et al. (2013) The subdural evacuation port system: Outcomes from a single institution experience and predictors of success. Clinical Neurology and Neurosurgery 115:658-664.
- Stanisic M, Lund JM, Mahesparan R (2005) Treatment of chronic subdural hematoma by burr-hole craniostomy in adults: influence of some factors on postoperative recurrence. ActaNeurochir (Wien) 147:1249-1256.
- Safain M, Roguski M, Antoniou A, Schirmer CM, Malek AM, et al. (2013) A single center’s experience with the bedside subdural evacuating port system: a useful alternative to traditional methods for chronic subdural hematoma evacuation. J Neurosurg 118:694-700.
- Lee L, Ker J, Ng HY, Munusamy T, King NK, et al. (2016) Outcomes of chronic subdural hematoma drainage in nonagenarians and centenarians: A multicenter study. J Neurosurg 124:546-551.
- Yamamoto H, Hirashima Y, Hamada H, Hayashi N, Origasa H, et al. (2003) Independent predictors of recurrence of chronic subdural hematoma: Results of multivariate analysis performed using a logistic regression model. J Neurosurg 98: 1217-1221.
- Mori K, Maeda M (2001) Surgical treatment of chronic subdural hematoma in 500 consecutive cases: clinical characteristics, surgical outcome, complications, and recurrence rate. Neurol Med Chir (Tokyo) 41:371-381.
- Parlato C, Guarracino A, Moraci A (2000) Spontaneous resolution of chronic subdural hematoma. SurgNeurol 53:312-317.
- Roger EP, Butler J, Benzel EC (2006) Neurosurgery in the elderly: brain tumors and subdural hematomas. ClinGeriatr Med 22: 623-644.
- Stippler M, Ramirez P, Berti A, Macindoe C, Villalobos N, et al. (2013) Chronic subdural hematoma patients aged 90 years and older. Neurol Res 35:243-246.
- Suzuki J, Takaku A (1970) Non-surgical treatment of chronic subdural hematoma. J Neurosurg 33:548-553.
- Leroy HA, Aboukais R, Reyns N, Bourgeois P, Labreuche J, et al. (2015) Predictors of functional outcomes and recurrence of chronic subdural hematomas. J ClinNeurosci 22:1895-1900.
- Honda Y, Sorimachi T, Momose H, Takizawa K, Inokuchi S, et al. (2015) Chronic subdural hematoma associated with disturbance of consciousness: significance of acute-on-chronic subdural hematoma. Neuro Res 37:985-992.
- Asghar M, Adhiyaman V, Greenway MW, Bhowmick BK (2002) Chronic subdural hematoma in the elderly–A North Wales experience. J R Soc Med 95: 290-292.
- Ramachandran R, Hegde T (2007)Chronic subdural hematomas-causes of morbidity and mortality. SurgNeurol 67:367-373.
- Sun TF, Boet R, Poon WS (2005) Non-surgical primary treatment of chronic subdural hematoma: Preliminary results of using dexamethasone. Br J Neurosurg 19: 327-333.
- Fujisawa H, Ito H, Kashiwagi S, Nomura S, Toyosawa M (1995) Kallikrein-kinin system in chronic subdural hematomas: Its roles in vascular permeability and regulation of fibrinolysis and coagulation. Journal of Neurology, Neurosurgery & Psychiatry 59:388-394.
- Wang D, Jiang R, Liu L, Dong JF, Zhang JN (2010) Membrane neovascularization and drainage of subdural hematoma in a rat model. Journal of Neurotrauma 27: 1489-1498
- Berghauser Pont LM, Dammers R, Schouten JW, Lingsma HF, Dirven CM (2012) Clinical factors associated with outcome in chronic subdural hematoma: A retrospective cohort study of patients on preoperative corticosteroid therapy. Neurosurgery 70:873-880.















