SPECT Scan and the Combined or "Fusion" SPECT-CT Images
|
| This new technology makes possible the visualization of lesions with a size <10 mm, due to its higher separation ability of the detecting apparatuses, and the correction of dispersed x-rays through interactive methods [1-7]. |
| The anatomical projections of the low dose (17-75 mA) CT are used for the attenuation of the SPECT images and the precise topographic localization of the pathological lesions with an abnormal accumulation of radio pharmaceuticals. The diagnostic height dose CT images (>80 mA) allows for the determination of the type of a morphological change of the visualized "hot" and "cold" lesions from the scintigraphy. This reflects on the reduced number of false-positive and false-negative results and therefore increasing the sensitivity and specificity of the scintigraphic studies. The nuclear medical part of the hybrid SPECT-CT images gives information for the functional activity of the primary neoplastic process of the breast and the secondary metastatic lesions, while the CT image is needed for determining the anatomical subtract of the visualized from the scintigraphy |
| "hot" lesions. The SPECT-CT exams find a different clinical value in breast cancer [8-17]. |
|
For the diagnosis of the primary tumor these exams have a limited role in the following cases
|
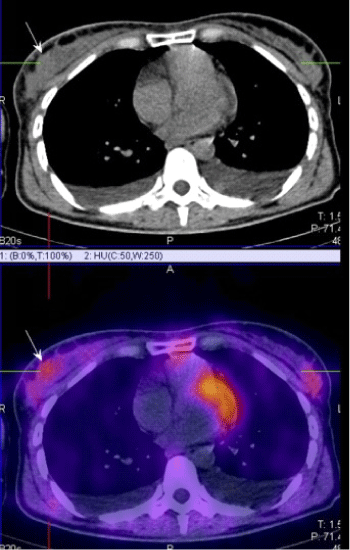
| 1. For the determination of the exact localization of a primary lesion from an unclear image obtained with the other visualizing methods (dense breasts, implants), with the need to perform a target biopsy (Figure 1) |
| 2. For the visualization of an occult primary tumor in patients with a metastatic process, and with clinical and laboratory data, pointing towards a breast origin |
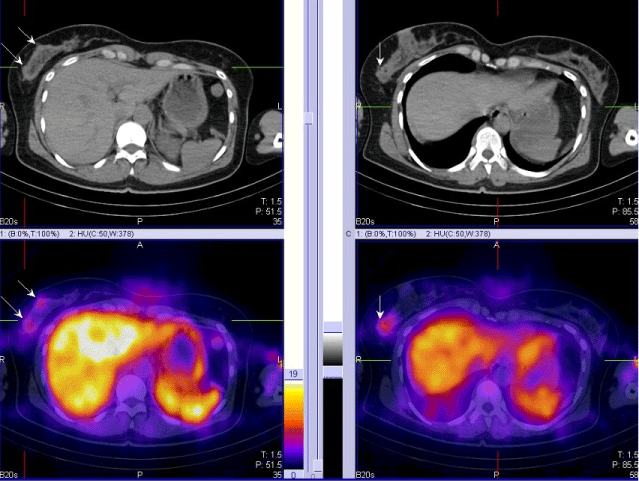
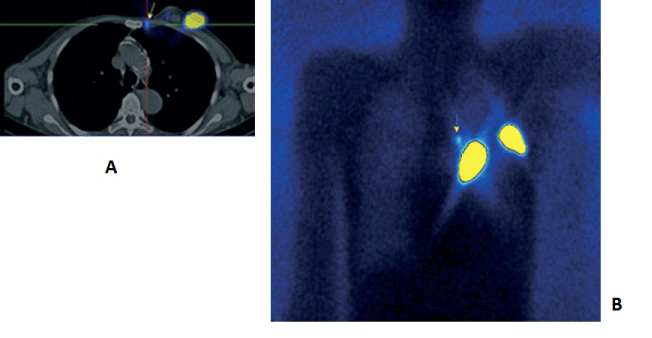
| 3. For visualizing multicentric and multifocal cancers (Figure 2). |
| SPECT-CT as a base line exam with 99mTc-MIBI/TF in local advancing tumors with a lined up neoadjuvant chemotherapy, for the purpose of determining the volume of the neoplastic formation and the intensity of the accumulated of the applied radio marker. Very low values of SUV (<2.2) of 18F-FDG have been noted in noninvasive and the mucinous histological variants of breast cancers, ductal carcinoma in situ, well differentiated lobular cancer, which are characterized by carbohydrate metabolism, similar to the one in normal cells [18,19]. |
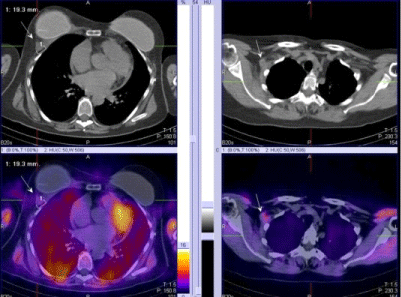
| 4. For the proper N/M staging of the disease in local advancing primary tumors of the breast and in inflammatory cancers (Figure 3). |
|
Preoperative N- staging of the disease
|
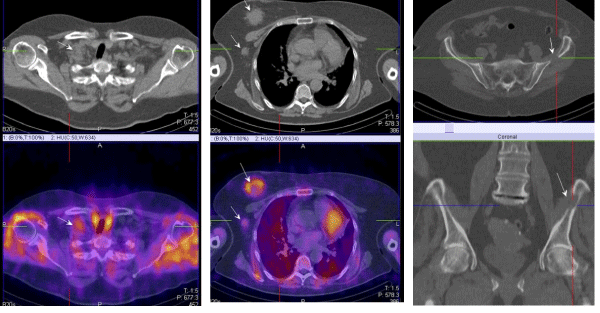
| The lymphoscintigraphic visualization of the sentinel lymph nodes (SLN) - detection and biopsy is a gold standard in the world's practice in the treatment of early stage breast cancers [20- 22]. In the last 5 years, intensively the purpose of the SPECT-CT examination has been researched along with the demonstration and the topography of the axillary SLN after a subcutaneous, intratumoral or a peri tumoral application of 99m??-Nanocoll, which is essential for their infra operative identification during the performance of a sentinel biopsy with a gamma probe [23,24]. This new technology improves the sensitivity of the conventional lymphoscintigraphy, which varieties between 72% and 94% up to 89%-100% due to the better resolution and contrast of the image [25,26], (Figures 4 and 5). |
| The identification of SLN sometimes leads to the establishment of atypical lymph drainage. In the literature we find data about parasternal lymph nodes which are seen in 20-25% of the patients after a peri tumoral injection of radio colloids; Intramammary - in 6%; Interpectoral - in 2%; supraclavicular - in 3% of the cases [25,26]. |
| In accordance to the literature [25,26] SPECT-CT exams increase the diagnostic sensitivity of the lymphoscintigraphic topogram in accordance to the visualization of: |
| • Additional "hot" SLN |
| • Visualization of ipsilateral parasternal lymph nodes |
| • SLN in cases of a negative planar lymphoscintigraphy |
| • Sub- and supraclavicular lymph nodes |
| • Prepectoral Intramammary SLN |
| • Interpectoral SLN |
| • Conjoining SLN on the planar image |
| • SLN in obese patients |
| • Increased in size, non palpable "cold" lymph nodes, located near SLN |
| Identification of parasternal SLN after a SPECT-CT has a clinical potential in medial and central localization of the primary tumors. The question about the intraoperative biopsy of these lymph nodes and the followed radiotherapy of this lymph pool is not yet resolved and it initiates different discussions. |
| The SPECT-CT studies can demonstrate exactly the intercostal space of the parasternal SLNs, which can be located and an intraoperative biopsy can be taken with an ?-detector or to get into the clinic tumoral volume in the radio therapeutic planning of high risk patients with central and medial localization. The identification of internal mammary SLNs is effected only in 75- 80% of patients in a routine lymphoscintigraphy, in comparison to >90% in axillary SLNs. [25,26]. One of the main reasons for this is the way of radio colloidal application. The visualization of axillary SLNs is obtained most often after a subdermal applicant above the tumor, with a volume of 0.2-0.3 ml, with an activity of 1-2 mCi. Scanning time is 45-90 min after injection. According to the literature, the visualization of internal mammary SLNs is done best after 2-4 deep parenchymal peri tumoral applications under ultrasound guidance, or after a single injection into the tumor with a volume of 0.3-0.5 ml, the detection is seen in 2-3 h after applying the radio colloid [23-25]. In a small percentage (2-3%) of the patients with medial and central localizations a secondary infiltration but only into the parasternal lymph nodes is seen (Figure 5). |
| PET-CT examination is not recommended as a standard procedure for N-staging of the axilla in early stage of breast cancer, due to the proven low sensitivity of the method, which is between 25% and 65% depending on the histological variant of the primary tumor and the respective lymphatic metastasis [27]. |
| SPECT-CT with 99mTc-MIBI/TF is recommended in locally advanced tumors and inflammatory cancers, due to the existing high risk for the process to disseminate, with an involvement of all three levels of the axillary lymph pools as well as the ipsilateral parasternal lymph nodes in a medial and central localization of the primary tumor [28,29]. |
|
Multimodal SPECT-CT methods have the highest clinical purpose for re-staging and follow-up in patients with breast cancer after the completion of complex therapy
|
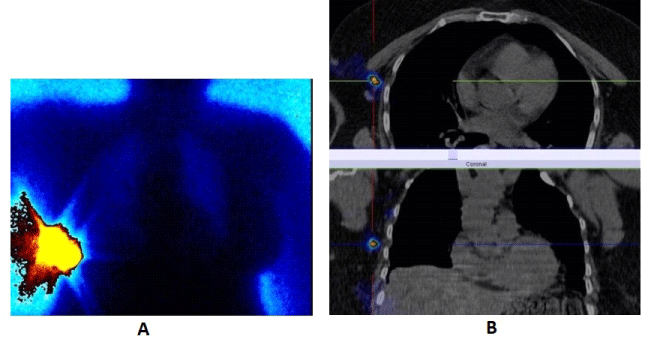
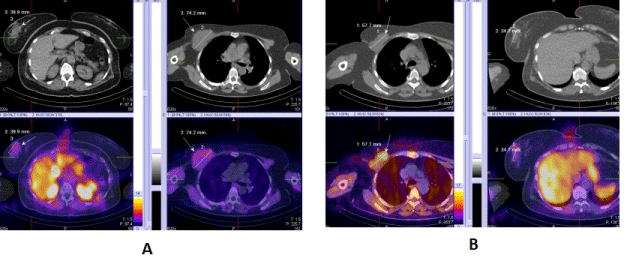
| SPECT-CT with 99mTc-MIBI/TF is used for finding active proliferative tumor tissue with accompanied clinical and biochemical data for a local recurrence in the region of the remaining breast parenchyma, around a breast implant, or on the chest wall after an organ sparing surgery, bilateral subcutaneous mastectomy with an augmentation or after a radical mastectomy for the purpose of re-staging the diseases [29,30] (Figure 6). |
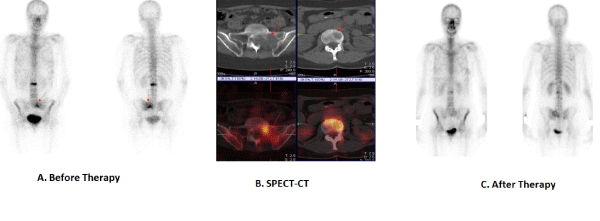
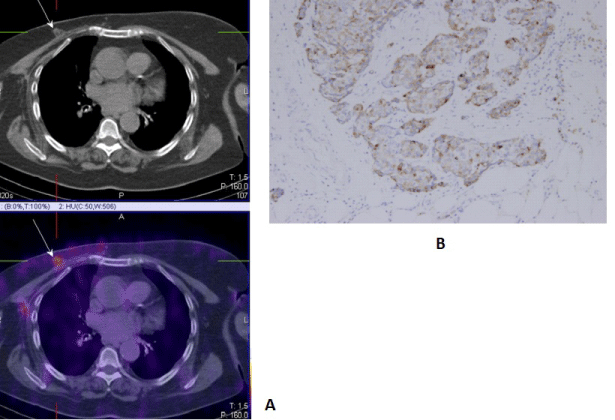
| For the diagnosis of a metastatic process and for determining it's level of dissemination with the presence of suspicions findings in a follow-up exam in patients with breast cancer. The module of choice for this purpose is the whole body bone scintigraphy followed by a target SPECT-CT exam in the zone of interest (Figure 7). The bone system is a predilection place for hematogeneous dissemination of this disease. The bone metastasis can be osteolytic, osteoblastic, or mixed [31,32]. |
| PET-CT with 18F-FDG is effective for visualizing the more aggressive osteolytic bone lesions and the intramedullar bone marrow lesions, while the osteosclerotic lesions are seen better on the bone scintigraphy after the application of 99mTc-MDP [33]. A higher sensitivity for the detection of tumor induced changes in the skeletal system is seen with the use of the new radio marker 18F-Fluoride in comparison with 18F-FDG. After a radionuclide therapy with 89Srontium or 153Samarium, or therapy with a bisphosphonate component the osteolytic lesions consolidate with a formation of an osteosclerotic peripheral valve and are of mixed character. |
| In positive scintigraphic findings with 99mTc-MDP of an uncertain character a SPECT-CT is performed to differentiate a potential benign diagnosis from a malignant process. Hybrid images are used localize a soft tissue component in some of the cases with direct bone and joint structural infiltration from a recurrence or metastatic formation, localized in the surrounding tissues (Figure 7). This is of great importance in the exact determination of the tumor volume in planning a stabilizing operative intervention or performing a palliative radiotherapy [31,32]. |
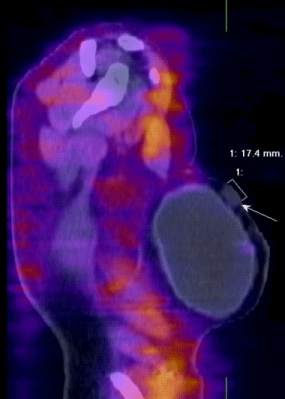
| In general, the multimodal nuclear medical methods with 99mTc- MIBI/TF can differentiate the active proliferating tumor tissue from post therapeutic fibrosis processes or physiological activity,therefore improving the diagnostic accuracy of the SPECT [29,30] (Figure 8). |
| For determining the effect of the performed therapy from the compared analysis, of the studies obtained from the baseline exam and the follow-up SPET-CT exams during the therapeutic process and at the end of the complex treatment - a full therapeutic response, partial remission, stable disease, and progression (Figure 9). With the hybrid methods two indicators can be used - the morphological parameters determined from the CT projections - number of lesions, size, localization and the degree of the accumulation of radio pharmaceuticals in "hot" spots on the SPECT images [29,30]. |
| For imaging of breast cancer recurrence in tumors containing neuroendocrine component with radiolabelled somatostatin analogues 111In-Ocrteoscan or 99mTc-Tektrotyd with increasing level of chromogranin A (Figure 10). |
|
Planning and radiotherapy
|
| SPECT-CT images have a practical use in determining the macroscopic (gross tumor volume–GTV) and the clinical (clinical tumor volume-CTV) targeted tumor volumes. Some patients demonstrate unsuspected tumor proliferative lesions, which increase the borders of the CT projection volumes, and in other cases reduction is needed in the predetermined borders, due to the differentiation of the fibrous tissue from the active metabolic tissue. This reflects on the priciest preparation of the individual radiotherapeutic plan in patients with breast cancer with the means of reducing the radio toxicity in a reached maximum therapeutic effect [32]. |
Conclusion
|
| The introduction of multimodal SPECT-CT methods is used for diagnosis, staging, and fallow-up for patients with breast cancer increased the diagnostic specificity and sensitivity of the nuclear medical diagnosis, which is of an important clinical value in determining and planning individual therapeutic approach in patients with breast cancer. |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
 |
 |
 |
 |
 |
| Figure 6 |
Figure 7 |
Figure 8 |
Figure 9 |
Figure 10 |
|
| |
| ` |















