Keywords
Febrile neutropenia (FN); MASCC; Solid tumors (ST); Haematological malignancies (HM); Absolute neutrophil count (ANC)
Introduction
FN is a medical emergency and is defined as a single oral temperature of greater than 38.3°C (101°F) or 38°C or greater (100°F) for over 1 hr in a patient with an absolute neutrophil count less than 500/cumm or less than 1,000/cumm, with predicted rapid decline [1,2]. FN causes economic loss, morbidity and mortality to patients [3].
IN 2000 International validation score was developed to identify the low risk FN by Multinational association of supportive care in cancer (MASCC). Risk stratification into low risk groups is required as these episodes can be treated on outpatient basis. This could help reduce the health care cost, morbidity and mortality [4].
There are a few studies on the profile of febrile neutopenia from India. This single center hospital based study was planned to evaluate the microbiological flora, MASCC score correlation and other clinical variables among febrile neutropenic patients in solid and hematological malignancies [5,6].
Materials and Methods
Source of data
A prospective, observational cohort study of consecutive febrile neutropenic episodes at Ramaiah hospitals over a period of 2 years, from October 2014 to September 2016. The aim of the study is to analyses the clinico-microbiological spectrum, evaluate the use of MASCC scoring index at Ramaiah hospital, Bangalore. IDSA guidelines are being followed for antibiotic policy in our institute.
Methods of data collection
Patients will be identified as per inclusion and exclusion criteria. History and Physical examination will be documented according to a standard Proforma.
Laboratory investigations
1. Complete blood count, urine routine.
2. Differential Counts and calculation of absolute neutrophil count.
3. Blood culture, urine culture, swabs for culture.
4. Chest X-ray radiograph.
5. HIV 1 and 2 testing by ELISA.
6. Each FN episode was given a MASCC score.
7. High-risk FN episode have a MASCC score <21 and were admitted to the hospital for empirical antibiotic therapy.
8. Low- risk FN episode have a MASCC score >21 and were on oral outpatient empirical antibiotic therapy.
Inclusion criteria
1. Histological diagnosis of malignancy.
2. FN due to chemotherapy.
3. ANC of <500 cells/mm3.
Exclusion criteria
1. HIV patients with cancer.
2. Age less than 16 years.
3. ANC: WBC X (% mature neutrophils + % bands)
4. MASCC score based on scoring system shown in Table 1.
| Characteristics |
Point Score |
| Burden of illness |
- |
| No or mild symptoms |
5 |
| Moderate symptoms |
3 |
| No hypotension |
5 |
| No chronic obstructive pulmonary disease |
4 |
| Solid tumour or no previous fungal infection in hematologic tumor |
4 |
| Outpatient status at the onset of fever |
3 |
| No dehydration |
3 |
| Age <60 years. |
2 |
Table 1: Score point to select patients.
Statistical analysis
All the quantitative variables such as age, MASCC score etc. were analyzed and expressed as mean and standard deviation.
All the qualitative variables such as characteristics of burden of illness, no or mild symptoms etc. were expressed in terms of percentage
Statistical analysis was done using the following tests:
1. Chi square test.
2. Correlation and coefficient.
3. ANOVA.
Results
Hundred FN episodes analyzed, of the 100 episodes 85 were in solid tumors, 15 were in hematological neoplasm. Female distribution was more (67% female and 33% men). Only 24% were aged above 60 years. Among solid tumors, breast cancer was most common (almost 27%) and among HT it was acute myeloid leukemia which was common shown in Figure 1.
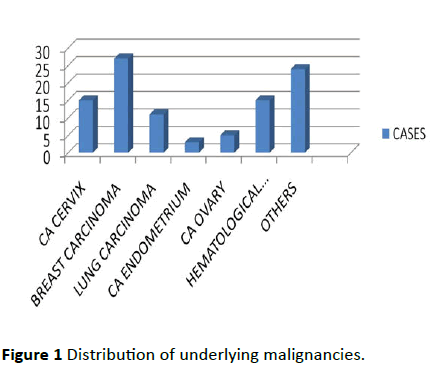
Figure 1: Distribution of underlying malignancies.
88% received were treated with 3rd generation cephalosporin. Oral treatment was given for 11 cases with ciprofloxacin or amoxicillin/claculunic acid. 23 cases out of 100 cases were given gram positive coverage either with vancomycin or teicoplanin based on the clinical/ microbiological indications.
Culture positivity was 25%. Urine being commonest culture positivity of 10%. Blood culture was positivity was 8%. Of the positive cultures 70% was gram negative species, 28% were gram positive Stapylococcus and two invasive Aspergilosis. Staphyloccous was the most common gram-positive species and E-coli, klebsilella was most common gram-negative species shown in Figure 2.
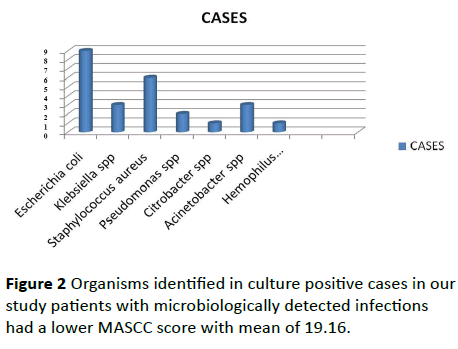
Figure 2: Organisms identified in culture positive cases in our study patients with microbiologically detected infections had a lower MASCC score with mean of 19.16.
The overall mortality was 18%. 12% in ST and 6% in HM. Significant higher mortality was observed in those episodes with positive cultures (17%). 40% of HM died as compared to 16.11% ST deaths.
In patients with MASCC score of >21 better response to treatment was observed in the study which showed very high statistical significance shown in Table 2.
| Response |
<21 |
>21 |
Total |
| <1 week |
0 |
23 |
23 |
| 1-2 weeks |
0 |
50 |
50 |
| Persistent for >3 weeks |
0 |
9 |
9 |
| Death |
18 |
0 |
18 |
| Total |
18 |
82 |
100 |
Table 2: Association between MASCC score and response (P - 0.001) In patients with MASCC score of >21 better response to treatment was observed in the study which showed very high statistical significance.
| Response |
Inpatient |
Outpatient |
Total |
| <1 week |
10 |
13 |
23 |
| 1-2 weeks |
19 |
31 |
50 |
| Persistent for >3 weeks |
3 |
6 |
9 |
| Death |
15 |
3 |
18 |
| Total |
47 |
53 |
100 |
Table 3: Association between status at the onset of fever and response. Febrile neutropenic episodes in admitted patients had a poor response compared to patients who were outpatients at the onset of fever. This showed statistical significance.
Presence of medical comorbidities at presentation also predicted poor outcome. High grade fever, fever >7 days, tachypnea, hypotension, renal failure, culture positivity, MASCC <21, ANC <50, febrile neutropenia episodes in inpatient (Table 3). All these predicted poor response (p - 0.001).
There is negative correlation between duration of neutropenia, fever and MASCC score. longer the duration of fever and neutropenia, lower MASCC score observed in Table 4. In patients with prolonged neutropenia, the duration of fever was also prolonged.
Table 4 Correlation between MASCC score, duration of neutropenia and duration of fever.
| Correlation |
|
Duration of neutropenia |
Duration of fever |
| MASCC Score |
r |
-0.466 |
-0.508 |
| p |
<0.001 |
<0.001 |
| N |
100 |
100 |
| Duration of neutropenia |
r |
- |
0.825 |
| p |
- |
<0.001 |
| N |
- |
100 |
Table 4: Correlation between MASCC score, duration of neutropenia and duration of fever.
Discussion
FN episodes are more common in haematological malignancies than solid tumors, following chemotherapy. The association of febrile neutropenia with acute leukemia was first demonstrated by Bodey.
Febrile neutropenia in Indian patients is published mostly in HM8-10. This prospective observational cohort study reports FN in both ST and HM. In our study, out of 100 cases of febrile neutropenia, 15 were haematological malignancies and 85 were solid tumors. Breast cancer was the commonest cancer. Acute myeloid leukemia was the commonest underlying haematological malignancies. In our study there were 33 males and 67 females with FN episodes. In our study culture positivity was 25%. In Arabian studies the culture positivity rate was 31% to 34% in mixed population of both ST and HM. Culture positivity in Indian patients among HM vary from 13% to 36% [7-10]. Staphylococcus was the most common grampositive isolate. This wide variability in culture positivity could be due to use conventional culture systems and wide spread use of empirical antibiotics in primary health care without culture sampling. E. coli was the commonest gram negative bacterial isolates in our study which is similar to other studies from India and developing countries [11,12]. Growth of Acinatobacter is of concern as it is associated with multidrug resistance. This is a major concerns in India due to inappropriate antibiotic usage causing drug resistance.
Talcot showed severity and duration of neutropenia, previous fungal infection, visceral organ involvement, organ dysfunction, uncontrolled malignancy, inpatient status at the onset of fever, hypotension, sepsis, co-morbidities including cardiovascular and pulmonary disease, leukemia or lymphoma diagnosis were associated with poor outcome in FN. Freifeld showed presence of abdominal pain, nausea and/or vomiting, diarrhoea, hemodynamic instability, neurological or mental changes, catheter-related infection, pulmonary infiltrates, renal failure, and liver insufficiency are associated with poor prognosis. In our study, patientsinpatient status at the onset of fever, ANC <50 Cumm3, demonstrable bacteraemia had poor outcome [13,14].
Swati et al. and Gupta et al. reported a mortality rate of 20.3% and 17.9% respectively in FN patients with HM from India. Recent studies report a wide range of mortality rate (7– 33%) in FN patients. The mortality in our study was 18% which is comparable to other studies. The incidence of fungal infection was like studies reported from India. Two cases of invasive aspergilosis was reported in HM, this may be due to construction work carried on during our study period [15-19].
Conclusion
FN episodes with tachypnea, hypotension, temperature >103°F, inpatient status at the onset of fever, ANC <50 Cumm3, deranged renal parameters and demonstrable bacteraemia had poor outcome in terms of recovery of ANC, mortality and length of hospital stay. The initial step should be risk stratification of patients using a validated risk assessment tool like the MASCC score. Early empirical intravenous antibiotic treatment and hospitalization though safe may lead to overtreatment and antibiotic resistance. Validation of simple additional parameters to identify low-risk FN episodes is required.
21344
References
- Bengre ML, Prabhu MV, Arun S, Prasad K, Bhat G (2012) Evaluation of the multinational association for supportive care in cancer (MASCC) score for identifying low risk febrile neutropenic patients at a south Indian Tertiary Care Centre. J Clin Diagn Res 6: 839-843.
- Gea-Banacloche JC, Palmore T, Walsh TJ, Holland SM, Segal BH (2008) Infections in the cancer patient. In: Devita, Hellmen, Rosenberg (eds). Devita, Hellman & Rosenberg’s Cancer: Principles & Practice of Oncology. (8th edn) Lippincott Williams & Wilkins, USA 21: 2579-2611.
- Sharma A, Lokeshwar N (2005) Febrile neutropenia in haematological malignancies. J Postgrad Med 51: 42-48.
- Talcott JA, Finberg R, Mayer RJ, Goldman L (1988) The medical course of cancer patients with fever and neutropenia: Clinical identification of a low risk subgroup at presentation. Arch Intern Med 148: 2561-2568.
- Jacob LA, Lakshmaiah KC, Govindbabu K, Suresh TM, Lokanatha D, et al. (2014) Clinical and microbiological profile of febrile neutropenia in solid tumors and hematological malignancies at a tertiary cancer care center in South India. Indian J Cancer 51: 464-468.
- Mathur P, Chaudhry R, Kumar L, Kapil A, Dhawan BA (2002) Study of bacteremia in febrile neutropenic patients at a tertiary-care hospital with special reference to anaerobes. Med Oncol 19: 267-272.
- Bodey GP, Buckley M, Sathe YS, Freireich EJ (1966) Quantitative relationships between circulating leukocytes and infections in patients with acute leukemia. Ann Inern Med 64: 328-334.
- Swati M, Gita N, Sujata B, Farah J, Preeti M (2010) Microbial etiology of febrile neutropenia. Indian J Hematol Blood Transfus 26: 49-55.
- Gupta A, Singh M, Singh H, Kumar L, Sharma A, et al. (2010) Infections in acute myeloid leukemia: An analysis of 382 febrile episodes. Med Oncol 27: 1037-1045.
- Kumar L, Kochupillai V, Bhujwala RA (1992) Infections in acute myeloid leukemia. Study of 184 febrile episodes. J Assoc Physicians India 40: 18-20.
- Prabhash K, Medhekar A, Ghadyalpatil N, Noronha V, Biswas S, et al. (2010) Blood stream infections in cancer patients: A single center experience of isolates and sensitivity pattern. Indian J Cancer 47: 184-188.
- Butt T, Afzal RK, Ahmad RN, Salman M, Mahmood A, et al. (2004) Bloodstream infections in febrile neutropenic patients: Bacterial spectrum and antimicrobial susceptibility pattern. J Ayub Med Coll Abbottabad 16: 18-22.
- Freifeld AJ, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, et al. (2011) Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clinical Infect Dis 252: e56-e93.
- Freifeld A, Marchigiani D, Walsh T, Chanock S, Lewis L, et al. (1999) A double-blind comparision of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 341: 305-311.
- Zahid KF, Hafeez H, Afzal A (2009) Bacterial spectrum and susceptibility patterns of pathogens in adult febrile neutropenic patients: A comparison between two time periods. J Ayub Med Coll Abbottabad 21: 146-149.
- Al-Ahwal MS, Al-Sayws F, Johar I (2005) Febrile neutropenia comparison between solid tumours and hematological malignancies. PAN Arab Med 2: 4-7.
- Sigurdardottir K, Digranes A, Harthug S, Nesthus I, Tangen JM, et al. (2005) A multi-centre prospective study of febrile neutropenia in Norway: Microbiological findings and antimicrobial susceptibility. Scand J Infect Dis 37:455-464.
- Horasan ES, Ersoz G, Tombak A, Tiftik N, Kaya A (2011) Bloodstream infections and mortality related factors in febrile neutropenic cancer patients. Med Sci Monit 17: CR304-309.
- Ghosh I, Raina V, Kumar L, Sharma A, Bakhshi S, et al. (2012) Profile of infections and outcome in high risk febrile neutropenia: Experience from a tertiary care cancer center in India. Med Oncol 29: 1354-1360.







