Research Article - (2022) Volume 10, Issue 6
Clinical Significance of Flow Cytometry Findings in Brazilian Patients with De Novo Acute Myeloid Leukemia
Linduarte Varela de Morais1#,
Rafael Duarte Lima2,4#,
Erica Aires Gil2#,
Aldair de Souza Paiva1,
Lenilton Silva da Silveira Junior2,
Victor de Lima Soares2,
Ciro Alexandre Merces Goncalves2,
Taissa Maria Moura de Oliveira2,
Dany Geraldo Kramer Cavalcanti e Silva3,
Gustavo Henrique de Medeiros Oliveira4 and
Geraldo Barroso Cavalcanti Junior2,4*
1Department of Clinical Medicine/ Federal University of Rio Grande do Norte (DMC/UFRN), Brazil
2Department of Clinical and Toxicological Analysis / Federal University of Rio Grande do Norte (DACT/UFRN), Brazil
3Department of Textile Engineering / Federal University of Rio Grande do Norte (DET/UFRN), Brazil
4Flow Cytometry Laboratory, Dalton Cunha Blood Center, Natal- Rio Grande do Norte, Brazil
#Equally contribution
*Correspondence:
Geraldo Barroso Cavalcanti Junior,
Department of Clinical and Toxicological Analysis / Federal University of Rio Grande do Norte (DACT/UFRN),
Brazil,
Fax: +55(84)32154226,
Email:
Received: 06-May-2022, Manuscript No. IPACLR-22-12774;
Editor assigned: 09-May-2022, Pre QC No. IPACLR-22-12774(PQ);
Reviewed: 23-May-2022, QC No. IPACLR-22-12774;
Revised: 17-Jun-2022, Manuscript No. IPACLR-22-12774(R);
Published:
24-Jun-2022, DOI: 10.36648/2386-5180.22.10.417
Abstract
Introduction: Immunophenotyping by Flow Cytometry (FC) is an essential method for diagnosis and classification of Acute Myeloid Leukemias (AML), and its extensive use could identify blast cell subpopulations with phenotypes rarely seen in normal myelopoiesis, correlating with clinical, morphological and prognostic characteristics.
Methods: In this study we analyzed 143 cases of AML, examining them for Leukemia-Associated Immunophenotype (LAIP) by FC immunophenotyping in leukemic cells using a panel of monoclonal antibodies (MoAb) for diagnosis and classification of Acute Leukemia (AL). At the same time, clinical, demographic and hematological data of these patients were also investigated. Most patients were male adults and splenomegaly and hepatomegaly were present in most cases.
Results: Immunophenotyping showed a characteristic profile of AML with expression of pan-myeloid antigens CD13, CD33 and Myeloperoxidase (MPO), combined with CD34 and CD117 in most cases. Expression of CD14 and CD64 were observed in AML with monocytic component (AML-M4/M5), CD235a, CD36 and CD71 in cases of erythroleukemia (AML-M6) and platelet glycoproteins CD41, CD42b and CD61 in acute megakaryocytic leukemia (AML-M7). Regarding the aberrant phenotype, higher levels of expression of CD4, CD7 and CD56 were observed, corresponding to 24.5%, 22.4%, and 16.1% of the cases, respectively.
Conclusion: We conclude that LAIP, as they are described here, were present in the vast majority of cases of investigated AML, with a relevant association with prognostic factors, clinical data, cytomorphological classification.
Keywords
Immunophenotyping, Flow cytometry, Monoclonal antibodies, Acute
myeloid leukemia, Leukemia-associated immunophenotype
Introduction
The characterization of Acute Leukemias (AL) is based on
multiparametric analysis which includes clinical features, cell
morphology, genetics and immunological markers [1,2]. These
parameters have been shown to be important for the diagnosis
and prognosis [1]. The first systems of classification of AL
were based on cytomorphological investigations enabling the
differentiation between Acute Myeloid Leukemia (AML) and
Acute Lymphoid Leukemia (ALL) [1-4].
The system established by the French-American British group
(FAB), established a morphological classification us AML according to the characteristics of the cells obtained from the
Bone Marrow (BM) aspirate in: AML-M0 (Undifferentiated AML);
AML-M1 (AML with minimal differentiation); AML-M2 (Maturing
AML); AML-M3 (Acute promyelocytic leukemia or APL); AML-M4
(Acute myelomonocytic leukemia), including the eosinophilic
form (AML-M4eos); AML-M5 (Acute monocytic leukemia),
subdivided into AML-M5a with predominance of monoblasts
and AML-M5b with predominance of promonocytes; AML-M6
(Erythroleukemia); AML-M7 (Acute megakaryoblastic leukemia)
[3,4].
Currently, cytomorphology remains a central tool in the
diagnostic and classification of hematological disorders [2]. It
should be integrated with other methods such as flow cytometry,
cytogenetics/molecular genetics and clinical data [1,2].
Flow Cytometry (FC) is widely used for diagnosis and monitoring
of hematological neoplasm, being a quicker and simpler
technique than other methods. Its high sensitivity and antigenic
quantification enables the identification of cells with Leukemia-
Associated Immunophenotype (LAIP) in the diagnosis and
detection of Minimal Residual Disease (MRD) through a panel of
Monoclonal Antibodies (MoAb) conjugated with fluorochromes
that recognize specific cell antigen epitopes, allowing a more
accurate delineation of the LAIP, enabling the differentiation
between AML and ALL [5-11].
Thus, the systematic use of MoAbs, such as CD13, CD33, CD65,
c-Kit receptor (CD117), antigen associated with hematopoietic
precursors (CD34), HLADR and anti-myeloperoxidase (MPO),
allow the definition of AML, being essential for the definition of
subtypes M0, M7, M5a and variant hypogranular form of APL
(AML-M3v) [10-15].
In addition, to contributing to the diagnosis, FC
immunophenotyping can also evidence blast cell heterogeneity
as reflected by the existence of a high variety of phenotypes as
well as detect antigen associations rarely seen in normal BM
cells, such as aberrant expression of lymphoid antigens and
asynchronous phenotypes [13].
To characterize the other FAB subtypes of AML, FC is less
important, but corroborates with the cytomorphological
findings in diagnosis and differentiation between the M1 and
M2 subtypes [10-13]. The expression of CD14 and CD64, are
present in the monocyte population, characterizing AML-M4/
M5 and erythroleukemia by the expression of glycophorin alpha
(CD235a) [6-8,13-15].
In order to assess the occurrence of LAIPs and correlate their
presence with the various morphological subtypes, we analyzed
143 de novo AML cases. Correlations between clinical data,
aberrant phenotypes, patient age and hematologic changes were
also examined.
Methods
Study Population
A total of 143 newly diagnosed AML cases at the Blood Center
Dalton Cunha (HEMONORTE), Natal City, located in Northeastern
Brazil in the period 2016-2018 were included in the study. Ethical approval for this study was granted by the Ethics and Research
Committees of the Onofre Lopes University Hospital of the Federal
University of Rio Grande do Norte (CAAE: 51300215.6.0000.5292).
All patients or their legal representatives signed the informed
consent form.
Demographic and clinical date for each case were obtained at
the time of diagnosis, including age, sex, fever, splenomegaly,
hepatomegaly, adenopathy, hemorrhagic phenomena and
presence of tumor masses. Hematologic information collected,
such as White Cell Count (WCC), platelets, hemoglobin and B12
vitamin measurement. In Peripheral Blood (PB). And BM smears
examination supplemented by cytochemical of Sudan Black
B (SBB) and MPO stained and FC immunophenotyping were
performed to confirm AML [16-20].
Hematological analysis from peripheral blood
and bone marrow
Hematologic information was performed in a hematological
analyzer (BC-3000 Plus, Myndray, China). Differential blood
cell counts were performed in PB smears stained with May-
Grüunwald-Giemsa (MGG-Laboclin, Brazil), in which a minimum
100 mononuclear cells were counted in an optical microscope
using 20x and 100x objective lenses (Zeiss Microscope, Gütting,
Germany) and the result of the cell count scored in percentage.
MGG-staining smears of BM were evaluated according to the FAB
criteria [3,4].
Serum dosage of vitamin B12 was performed by
chemiluminescence immunoassay (ARCHITECT i1000SR
immunoassay analyzer, Abott, USA) with the aim of excluding
Megaloblastic Anemia (MA) in patients with suspected AML-M6.
Immunophenotyping studies
Erythrocyte-lysed whole BM samples were analyzed by FC using
a large panel of MoAbs in four combinations (Table 1) [17-20].
Detection of surface, cytoplasmic (cyt) such as MPO, cytCD13,
cytCD79a, cytCD22, cytCD3, anti-IgM and nuclear (nu) Terminal
Deoxynucleotidyl Transferase (TdT) antigens were performed
following a previously established protocol [19-23].
| MoAb/Fluorochromes |
Clone |
Reactivity |
Source |
| CD45APC |
HI3 |
Common leukocyte antigen |
BD |
| HLADRAPC |
L243 |
MHC class II cell surface receptor |
BD |
| CD34PerCP |
8G12 |
Immature hematopoetic precursors |
BD |
| CD117APC |
CE/IVD |
c-kit receptor / Myeloid precursor |
BD |
| anti-MPOFICT |
CLB |
Myeloperoxidase / Myeloid antigen |
BD |
| CD13FICT |
L13 |
Aminopeptidase N / Myeloid antigen |
BD |
| CD33PE |
P67.6 |
Myelomonocytic antigen / Myeloid precursors |
BD |
| CD36PE |
NLO7 |
Scavage receptor |
BD |
| CD14PerCP |
M5E2 |
Lipopolysaccharide receptor / monocytes antigen |
BD |
| CD64APC |
MB22 |
Precursor monocytes antigen |
BD |
| CD65FICT |
88H |
Myeloid antigen / Myeloid precursors |
BD |
| CD66bFICT |
G10F5 |
Carcinoembryonic antigen / myeloid antigen |
BD |
| CD71FICT |
L01.1 |
Transferrin receptor |
BD |
| CD235aFICT |
GA-R2 |
Glycophorin A / eritroid antigen |
BD |
| CD41FICT |
HIP8 |
Glicoprotein IIb/ Platelet and platelet precursors |
BD |
| CD42bFICT |
HIP1 |
Glicoprotein IX / Platelet and platelet precursors |
BD |
| CD61PerCP |
RUU-PLF12 |
Glicoprotein IIIa / Platelet and platelet precursors |
BD |
| CD1aPerCP |
HI-149 |
Thymocytes antigens / T-lymphocytes precursors |
BD |
| CD2FICT |
S5.2 |
T-lymphocytes mature and precursors |
BD |
| CD3APC |
UCHT1 |
Best marker for cells of T lineage |
BD |
| CD7APC |
M-T701 |
T-cell lineage-associated antigen |
BD |
| CD56PE |
B159 |
Natural Killer Cells |
BD |
| CD10FICT |
HI10 |
CALLA antigen / B-lymphocytes precursors |
BD |
| CD19PerCP |
89B |
Pan-B lineage lymphocytes |
BD |
| CD20FICT |
2H7 |
Pan-B lineage lymphocytes |
BD |
| CD22FICT |
HIB22 |
Pan-B lineage lymphocytes |
BD |
| CD79aPE |
HM47 |
Early B-lymphocytes precursors |
BD |
| IgMAPC |
G20-123 |
IgM immunoglobulin heavy chain |
BD |
| TdTPE |
HT9 |
Terminal-deoxynucleotidyl Transferase |
BD |
| Multitest conjugated MoAb |
BD/Simultest Leucogate: CD14FICT/CD45PE: CD14 mature monocites and CD45 common leukocyte antigen;
BD/Oncomark CD14FICT/CD64PE: Monocytic development;
BD/Oncomark CD7FICT/CD33PE: CD7 T-lymphocytes and CD33 myelomonocytic antigen; BD/Oncomarck /CD15FICT/CD34PE: CD15 mature granulocytes and CD34 Immature precursors; BD/Multitest 4-Color: CD3FICT/CD8PE/CD45PerCP/CD4AP: T-subsets lymphocytes;
BD/Multitest 4-Color: CD3FICT/CD16-56PE/CD45PerCP/CD19AP: T, NK and B lymphocytes |
| IgG1FITC/IgG1PE/IgG1PerCP/IgG1APC: BD Isotypic matched antibodies |
| Note: Monoclonal antibodies (MoAb); Fluorescein isothiocyanate (FITC), Phycoerythrin (PE), Peridinin-chlorophyll protein (PerCP), Allophycocyanin (APC), Becton & Dickinson (BD). |
Table 1: Monoclonal antibodies used in this study.
Data acquisition and analysis were performed on a FACScalibur
flow cytometer (Becton Dickinson Immunocytometry Systems,
San José, CA, USA) using Cell-Quest software. Calibration and
fluorescence compensation were carried out using Calibrite
beads (Becton Dickinson, San José, CA, USA) and immunoglobulin
isotype-matched negative controls. Identification of blast cells
was performed using Forward Scatter (FSC) versus Side Scatter
(SSC) parameters and/or CD45 intensity versus SSC dot plots.
Antigen expression was considered to be positive when the
percentage of positive blast cells was equal or greater than 20%
[19,20].
Results
Out of the 143 cases, 85 males and 58 females were studied. Patients were aged between 02 and 92 years. The age group most affected by the disease was 20 to 59 years (65 cases), followed by individuals over the age of 60 with 52 patients. All cases were classified according to FAB criteria and FC immunophenotyping distributed into: 03 AML-M0, 53 AML-M1, 22 AML-M2, 09 AML-M3, 27 AML-M4, 14, AML-M5a, 08 AML-M5b, 04 AML-M6 and 3 AML-M7 (Table 2).
| Date |
Patients
n=143 |
AML-M0
n= 03
n+ (%) |
AML-M1
n= 53
n+ (%) |
AML-M2
n= 22
n+ (%) |
AML-M3
n= 09
n+ (%) |
AML-M4
n= 27
n+ (%) |
AML-M5a
n= 14
n+ (%) |
AML-M5b
n= 08
n+ (%) |
AML-M6
n= 04
n+ (%) |
AML-M7
n= 03
n+ (%) |
Gender
Male
Female |
85
58 |
02 (66.7)
01 (33.3) |
31 (58.5)
22 (41.5) |
13 (59.1)
09 (40.9) |
07 (77.8)
02 (22.2) |
17 (63.0)
10 (37.0) |
07 (50.0)
07 (50.0) |
06 (75.0)
02 (25.0) |
01 (25.0)
03 (75.0) |
01 (33.3)
02 (66.7) |
Age (Years)
= 10
>10- 19
>20- 59
= 60 |
14
12
65
52 |
00 ( - )
00 ( - )
03 (100)
00 ( - ) |
08(15.1)
04 (07.5)
17 (32.1)
24 (45.3) |
02 (09.1)
04 (18.2)
12 (54.5)
04 (18.2) |
00 ( - )
02 (22.2)
06 (66.7)
01 (11.1) |
00 ( - )
02 (07.4)
14 (51.9)
11 (40.7) |
01 (07.1)
00 ( - )
06 (42.9)
07 (50.0) |
02 (25.0)
00 ( - )
03 (37.5)
03 (37.5) |
00 ( - )
00 ( - )
03 (75.0)
01 (25.0) |
01 (33.3)
00 ( - )
01 (33.3)
01 (33.3) |
Clinical date
Splenomegaly
Hepatomegaly
Bone pain
Fever
Bleeding
Gingival hypertrophy
Tumor mass
Chloroma
Lymphadenopathy |
118
99
40
25
21
19
15
09
08 |
03 (100)
03 (100)
03 (100)
03 (100)
01 (33.3)
00 ( - )
00 ( - )
00 ( - )
00 ( - ) |
45 (84.9)
39 (73.6)
13 (24.5)
15 (28.3)
05 (09.4
00 ( - )
00 ( - )
03 (05.7)
05 (09.4) |
19 (86.4)
16 (72.7)
07 (31.8)
02 (09.1)
00 ( - )
00 ( - )
00 ( - )
04 (18.2)
01 (04.5) |
00 ( - )
02 (22.2)
00 ( - )
00 ( - )
09 (100)
00 ( - )
00 ( - )
00 ( - )
00 ( - ) |
26 (96.3)
17 (63.0)
05 (18.5)
01 (03.7)
02 (07.4)
04 (14.8)
03 (11.1)
02 (07.4)
01 (03.7) |
14 (100)
13 (92.9)
05 (35.7)
01 (07.1)
04 (28.6)
10 (71.4)
11 (78.6)
00 ( - )
01 (07.1) |
07 (87.5)
05 (62.5)
04 (50.0)
02 (25.0
00 ( - )
05 (62.5
01 (12.5)
00 ( - )
00 ( - ) |
02 (50.0)
03 (75.0)
02 (50.0)
00 ( - )
00 ( - )
00 ( - )
00 ( - )
00 ( - )
00 ( - ) |
02 (66.7)
01 (33.3)
01 (33.3)
01 (33.3)
00 ( - )
00 ( - )
00 ( - )
00 ( - )
00 ( - ) |
| Note: (FAB) French American [3-5] |
Table 2: Correlation between demographic and clinical date and FAB subgroups M0 into M7 of patients with acute myeloid leukemia.
Clinical data associated with the AML were present in most cases. Of these, splenomegaly was predominant with 118 cases, followed by hepatomegaly and bone pain with 99 and 40 cases respectively. Hemorrhagic phenomena were predominantly in APL and directly associated with low platelet count in PB. Presence of gingival hypertrophy and tumor mass were predominant in AML-M5a (Table 2).
| Date |
Patients
n=143 |
AML-M0
n= 03
n+ (%) |
AML-M1
n= 53
n+ (%) |
AML-M2
n= 22
n+ (%) |
AML-M3
n= 09
n+ (%) |
AML-M4
n= 27
n+ (%) |
AML-M5a
n= 14
n+ (%) |
AML-M5b
n= 08
n+ (%) |
AML-M6
n= 04
n+ (%) |
AML-M7
n= 03
n+ (%) |
B12 vitamin (pg/mL)
= 200
> 200 |
Ur
Ur |
Ur
Ur |
Ur
Ur |
Ur
Ur |
Ur
Ur |
Ur
Ur |
Ur
Ur |
Ur
Ur |
00 ( - )
04 (100) |
Ur
Ur |
| + Cytochemistry (SBB/MPO) |
132 |
00 ( - ) |
53 (100) |
22 (100) |
09 (100) |
27 (100) |
13 (92.9) |
08 (100) |
* 00 ( - ) |
*00 ( - ) |
WBC /PB(x 103/?L)
= 5.0
>5.0-10
> 10 - 50
> 50 |
10
11
40
82 |
00 ( - )
00 ( - )
00 ( - )
03 (100) |
01 (01.9)
01 (01.9)
16 (30.2)
36 (67.9) |
00 ( - )
05 (22.7)
06 (27.2)
11 (50.0) |
08 (88.9)
00 ( - )
00 (- )
01 (11.1) |
00 ( - )
02 (07.4)
11 (40.7)
14 (51.9) |
00 ( - )
01 (07.1)
03 (21.4)
10 (71.4) |
00 ( - )
00 ( - )
01 (12.5)
06 (75.0) |
01 (25.0)
01 (25.0)
00 ( - )
02 (50.0 |
00 ( - )
01 (33.3)
02 (66.7)
01 (33.3) |
% Blastic cells (PB)
= 50
>50 |
26
117 |
00 ( - )
03 (100) |
12 (22.6)
41 (77.4) |
04 (18.2)
18 (81.8) |
01 (11.1)
08 (88.9) |
04 (14.8)
23 (85.2) |
01 (07.1)
13 (92.9) |
02 (25.0)
06 (75.0) |
01 (25.0)
03 (75.0) |
01(33.3)
02 (66.7) |
Platelet count/PB (x 103/?L)
= 20
>20- 50
50 - 100
> 100 |
17
63
53
10 |
00 ( - )
02 (66.7)
01 (33.3)
00 ( - ) |
04 (07.5)
31 (58.5)
15 (28.3)
03 (05.7) |
00 ( - )
11 (50.0)
10 (45.5)
01 (04.5) |
08 (88.9)
01 (11.1)
00 ( - )
00 ( - ) |
02 (07.4)
08 (29.6)
15 (55.6)
02 (07.4) |
02 (14.3)
06 (42.8)
05 (37.1)
01 (07.1) |
01 (12.5)
01 (12.5)
04 (50.0)
02 (25.0) |
00 ( - )
03 (75.0)
01 (25.0)
00 ( - ) |
00 ( - )
00 ( - )
02 (66.7)
01 (33.3) |
Hemoglobin (g/dL)
< 10.0
10.0 - 12.0
> 12.0 |
107
33
03 |
02 (66.7)
01 (33.3 )
00 ( - ) |
45 (84.9)
08 (15.1)
00 ( - ) |
13 (59.1)
08 (36.4)
01 (04.5) |
08 (88.9)
01 (11.1)
00 ( - ) |
16 (59.3)
09(33.3)
02 (07.4) |
11 (78.6)
03 (21.4)
00 ( - ) |
06 (75.0)
02 (25.0)
00 ( - ) |
03 (75.0)
01 (25.4)
00 ( - ) |
03 (100)
00 ( - )
00 ( - ) |
| Note: (FAB) French American British classification 3-5; (+) Positive Reaction; (n) number of patients tested; (n+) number of positive cases; (%) percentage of positive cases; (PB),peripheral blood; (WBC) white cell count; (SBB) Sudan Black B; (MPO) Myeloperoxidase; (*) SBB/MPO staining negative in erythroid and megakaryocyte population; (Ur)unrealized. |
Table 3: Correlation between hematological date and FAB subgroups M0 to M7 of patients with acute myeloid leukemia.
A WBC variation was observed, which ranged from 700 to 299.000/μL. There was a predominance of patients with WBC more than 50.000/μL with 82 cases, followed by 40 cases with WBC between more than 10.000 and 50.000/μL. Patients with WBC between 5.000 and 10.000/μL and less than 5.000/μL were observed in 11 and 10 cases, respectively. Hemoglobin levels less than 12.0g/dL and thrombocytopenia were observed in most cases, with more pronounced results in cases of APL. Cytochemical stain of SBB and MPO were positive in most cases, notably in subgroups M1, M2 and M3, and negative in AML-M0, M6 and M7 (Table 3).
| AcMo |
Patients
n=143
n+ (%) |
AML-M0
n= 03
n+ (%) |
AML-M1
n= 53
n+ (%) |
AML-M2
n= 22
n+ (%) |
AML-M3
n= 09
n+ (%) |
AML-M4
n= 27
n+ (%) |
AML-M5a
n= 14
n+ (%) |
AML-M5b
n= 08
n+ (%) |
AML-M6
n= 04
n+ (%) |
AML-M7
n= 03
n+ (%) |
| CD45 |
138 (96.5) |
03 (100) |
53 (100) |
22 (100) |
09 (100) |
27 (100) |
14 (100) |
08 (100) |
00 ( - ) |
01 (33.3) |
| HLADR |
120 (83.9) |
03 (100) |
53 (100) |
22 (100) |
00 ( - ) |
27 (100) |
14 (100) |
08 (100) |
02 (50.0) |
01 (33.3) |
| CD34 |
106 (74.1) |
03 (100) |
53 (100) |
12(54.5) |
00 ( - ) |
18 (66.7) |
14 (100) |
01 (12.5) |
04 (100) |
01 (33.3) |
| CD117 |
131 (91.6) |
02 (66.7) |
53 (100) |
20 (90.1) |
09 (100) |
27 (100) |
13 (92.9) |
05 (62.5) |
02 (50.0) |
01 (33.3) |
| anti-MPO |
124 (86.7) |
03 (100) |
53 (100) |
10 (100) |
09 (100) |
27 (100) |
05 (35.7) |
08 (100) |
00 ( - ) |
00 ( - ) |
| CD13 |
130 (90.1) |
03 (100)* |
53 (100) |
22 (100) |
09 (100) |
27 (100) |
14 (100) |
07 (87.5) |
00 ( - ) |
00 ( - ) |
| CD33 |
139 (97.2) |
01 (33.3) |
53 (100) |
22 (100) |
09 (100) |
26 (96.3) |
14 (100) |
08 (100) |
04 (100) |
03 (100) |
| CD36 |
44 (30.8) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
25 (92.6) |
09 (64.3) |
06 (37.5) |
04 (100) |
03 (100) |
| CD14 |
38 (26.6) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
22 (81.5) |
10 (71.4) |
06 (75.0) |
00 ( - ) |
00 ( - ) |
| CD64 |
46 (32.2) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
26 (96.3) |
14 (100) |
06 (75.0) |
00 ( - ) |
00 ( - ) |
| CD65 |
118 (82.5) |
00 ( - ) |
53 (100) |
22 (100) |
09 (100) |
27 (100) |
07 (50.0) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD66b |
49 (34.3) |
00 ( - ) |
00 ( - ) |
22 (100) |
00 ( - ) |
27 (100) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD71 |
42 (29.4) |
03 (100) |
20 (37.7) |
05 (22.7) |
00 ( - ) |
00 ( - ) |
05 (35.7) |
00 ( - ) |
04 (100) |
00 ( - ) |
| CD235a |
04 (02.8) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
04 (100) |
00 ( - ) |
| CD41 |
03 (02.1) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
03 (100) |
| CD42b |
03 (02.1) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
03 (100) |
| CD61 |
03 (02.1) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
03 (100) |
| CD1a |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD2 |
10 (06.7) |
00 ( - ) |
04 (02.0) |
04 (02.0) |
00 ( - ) |
02 (07.4) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD3 |
08 (05.6) |
00 ( - ) |
02 (03.8) |
03 (13.6) |
00 ( - ) |
02 (07.4) |
01 (07.1) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD3+/CD4+ |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD3+/CD8+ |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD4 |
35 (24.5) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
20 (70.1) |
11 (78.6) |
04 (50.0) |
00 ( - ) |
00 ( - ) |
| CD7 |
32 (22.4) |
02 (66.7) |
20 (37.7) |
05 (22.7) |
00 ( - ) |
03 (11.1) |
01 (07.1) |
00 ( - ) |
00 ( - ) |
01 (33.3) |
| CD56 |
23 (16.1) |
00 ( - ) |
03 (05.7) |
11 (50.0) |
02 (22.2) |
00 ( - ) |
05 (35.7) |
02 (25.0) |
00 ( - ) |
00 ( - ) |
| CD10 |
01 (0.70) |
00 ( - ) |
01 (01.9) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD19 |
12 (08.4) |
00 ( - ) |
02 (03.8) |
09 (40.9) |
00 ( - ) |
01 (03.7) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD20 |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| CD22 |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| cytCD79a |
04 (02.8) |
00 ( - ) |
02 (03.8) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
02 ( - ) |
02 (25.0) |
00 ( - ) |
00 ( - ) |
| IgM |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| nuTdT |
04 (02.8) |
00 ( - ) |
04 (07.5) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
00 ( - ) |
| Note: (FAB) French American British classification 3-5; (n) number of patients tested; (n+) number of positive cases; (%) percentage of positive cases; (HLADR) Type II major histocompatibility complex; (anti-MPO) AcMo against myeloperoxidase; (CD13* or cytCD13) Intracytoplasmic CD13; (CD3+/CD4+) T-helper lymphocytes; (CD3+/CD8+) T-cytotoxic lymphocytes; (cytCD79a) Intracytoplamatic CD79a; (nuTdT) nuclear Terminal deoxinucleotidil Transferase. |
Table 4: Diagnosis and classification of AML based on reactivity with various monoclonal antibodies.
In FC analysis, leukemic cells were initially identified by FSC/ SSC ratio and expression of pan-myeloid antigens: CD13, CD33, CD65, CD117 and MPO in most cases. CD34 was expressed in most cases, characterizing the presence of blast cells with little differentiation (Table 4 and Figure 1).
Leukemic cells from APL showed a characteristic pattern of positivity to CD13, CD33, MPO and CD117 and negativity for CD34 and HLADR (Figure 1). Monocytic AML (M4/M5), expression of CD14 and CD64, associated with CD13, CD33 and HLADR (Figures 1).
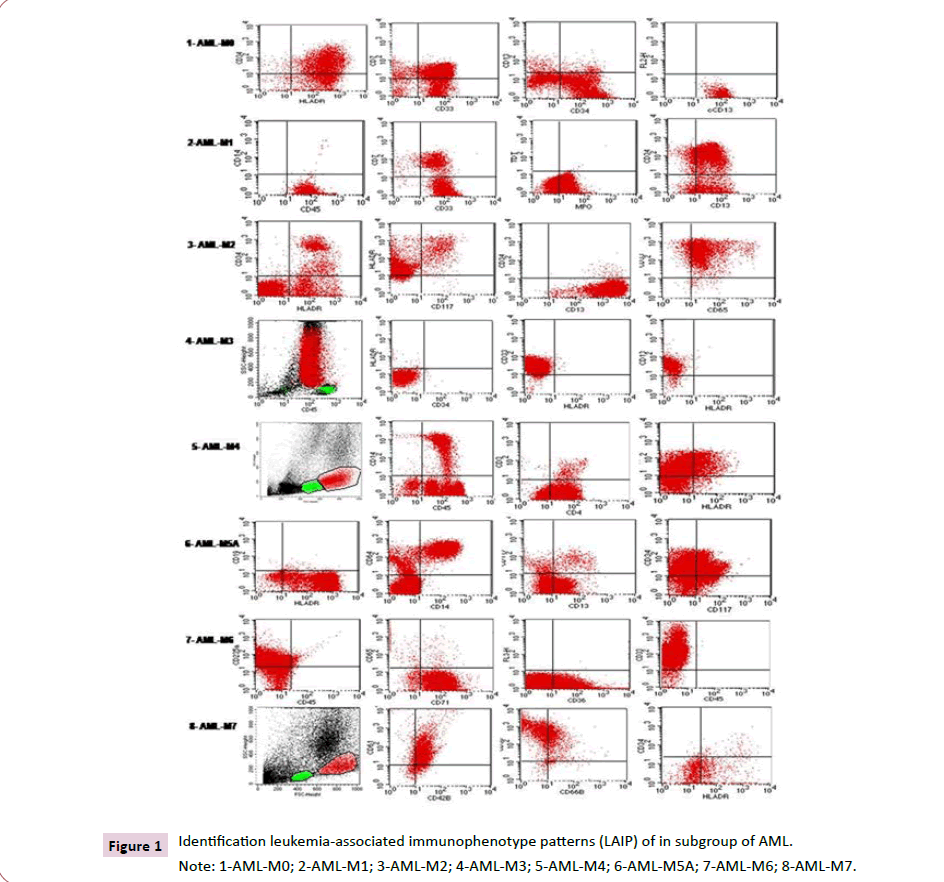
Figure 1:Identification leukemia-associated immunophenotype patterns (LAIP) of in subgroup of AML.
Note: 1-AML-M0; 2-AML-M1; 3-AML-M2; 4-AML-M3; 5-AML-M4; 6-AML-M5A; 7-AML-M6; 8-AML-M7.
AML-M6 was identified by strong expression of CD235a and CD71, associated with CD33, CD36 and CD34 (Figure 1). In these AML, serum levels of vitamin B12 were normal, ruling out the diagnosis of MA.
AML-M7 was confirmed by the expression of platelet glycoproteins CD41, CD42b and CD61 (Figure 1).
Aberrant expression of lymphoid antigens has been observed in some cases, especially CD4, CD7 and CD56. The CD4 was observed in 24.5% of cases, more present in cases of AML-M4/ M5. CD7 was positive in 22.4% of cases, with more expressive values in AML-M0/M1 and CD56 was expressed in 16.1% of cases, notably in the subtypes AML-M2, M5a/b and M3. Other lymphoid antigens such as TdT, CD3, CD10, CD19, CD2 and CD79a were observed in less frequently.
Discussion
For the diagnosis and classification of AML, the World Health
Organization (WHO) recommends careful integration of clinical
history, morphology analysis of BM and PB smears, cytogenetic/
molecular genetic analysis and immunophenotyping [24].
However, cytogenetic/molecular analyzes may not be available
in many services, and cytomorphological analysis complemented
by immunophenotyping is satisfactory for rapid diagnosis and
classification of this leukemias [25].
It is important to identify the subtype of AML, as this influences
the choice of treatment type and the patient's prognosis 26. For
example, in the case of APL, in which the diagnosis is clinically urgent, the combination of these two modalities permits a
diagnosis with high precision [1,16,26]. In some other types of
AML, immunophenotypic features may provide an indication as
to the cytogenetic anomaly likely to be present [1, 6,17,18,26].
Highlighting the AML with t(8;21) observed in immunophenotype
cases with strong CD34+, HLADR+, weak CD33+, MPO+, CD65+
or CD15+ and IL-2 receptor (CD25+) can observe aberrant
expression for lymphoid antigens CD19, CD79a, TdT and CD56.
In AML, chromosome 16 inversion correlates with expression of
CD34, CD117 and myeloid subpopulations with MPO+, CD13+,
CD33+, CD15+, CD65+; AML with a monocytic component with
expression of CD11b, CD11c, CD64, CD36 and CD14 and aberrant
expression of CD4 AND CD2. AML with t(9;11) 11q23 correlates
with aberrant expression of the lymphoid antigens CD19 and CD2
[1,6,-18,26].
Detection of MPO is probably the most specific technique for
differentiating between myeloid and lymphoid lineages, which
can be done by cytochemical methods or immunophenotyping
[16,25]. It is a lysosomal enzyme found in the primary granules
of cells in the myeloid series [26]. Its expression occurs at the
beginning of myeloid differentiation and appears to be specific
for cells of that lineage. MPO has never been reported in ALL
[2,7,27,28].
Most studies have found a higher incidence of AML in males,
although this predominance is not as distinct as in ALL [1]. In our
series, a male prevalence was present, with a male to female
ratio of 1.4 to 1 (Table 2).
Most patients had pallor and fatigue, possibly due to anemia [1].
In present study, splenomegaly and hepatomegaly were the most
common clinical findings. Bleeding was more present in patients
with APL (Table 2).
In the immunophenotypic evaluation of AL, the expression of one
or more pan-myeloid antigens such as CD13, CD33, CD65, CD117
and MPO are sufficient for the diagnosis of AML [1,13,17-19,26].
Furthermore, the immunophenotypic classification has
diagnostic and prognostic importance in some subtypes of AML.
Thus, it is essential for the diagnosis of AML-M0 and M7, being
helpful in the diagnosis of the variant hypogranular form of APL
(AML-M3v), in the differentiation between subgroups AML-M1
and M2, in subtypes AML-M5a and M5b, and in the differential
diagnosis between AML-M6 and MA [6,13-15,18,19,25].
The AML-M0 is characterized by the infiltration of BM by blast
cells with negative cytochemical reaction for MPO and SBB.
Blasts cells are small, with loose chromatin and evident nucleoli,
presenting agranular cytoplasm, without Auer bodies [2-4].
Immunophenotyping shows a blast population with a low FSC/
SSC ratio, with positivity for at least one of the myeloid antigens
such as CD33, cytCD13, CD117 and CD34 24-25. Lymphoid
lineage antigens are generally negative, although CD7 and
CD56 are observed in some cases [1,25]. As the therapeutic
approach to AML differs from ALL, it is important to perform
immunophenotyping in the differentiation between AML-M0
from ALL and the consequent correct treatment guidance
[1,25].
The AML-M1 is associated with the expression of CD13, CD33,
CD34, CD65, CD117 and HLADR in variable combinations [25].
In this AML group, the co-expression of CD34 and HLADR is
significantly greater than that observed in the AML groups with
maturation as M2/M3 [1,6,13,17,18,25].
AML-M2 is characterized by the presence of >30% of myeloblasts
associated with 10% of mature granulocytes in BM. Blast cells
of a large size, with abundant and basophilic cytoplasm, often
containing azurophilic granules. Auer bodies are frequent [3-4].
Immunophenotyping leukemic cells exhibit CD65, CD66, HLADR,
CD13 and CD33, but CD34 expression is very weak or sometimes
may be absent [1,6,13,18,19,25]. Aberrant expression of
lymphoid antigens CD19, CD2, CD7 and natural killer cells (CD56)
can also be observed, as these are associated with t (8:21) and
are related to good prognosis and higher rates of complete
prolonged remission in adult patients [25].
APL is defined by the proliferation of leukemic promyelocytes
in BM, which have a large nucleus, and a cytoplasm with many
coarse granulations. In some cases, the presence of numerous
Auer bodies "faggot cell" is observed [3-4,16]. In AML-M3v,
promyelocytes have a large and convoluted nucleus. The
cytoplasm is basophilic with little or no granulation [3,4].
Cytochemical stains for MPO and SBB are strongly positive in
both types of APL [16].
The immunophenotypic reveals high auto fluorescence and
higher FSC/SSC ratio [24] and positivity to MPO, CD13, CD33 and
CD117, but there is a lack of CD34 and HLADR [15,17,18,25]. These
immunophenotypic, clinical and hematological characteristics of
APL were also found in the present study (Tables 2-4 and Figure 1).
In acute myelomonocytic leukemia (AML-M4), leukemic cells
exhibit monocytic and granulocyte precursors [3-4]. Monocytic
precursors constitute >20% of the nucleated cells in the BM,
representing about 12% of AML [3-4]. The variant eosinophilic
form (AML-M4eo) can be found, with an increase in the number
of eosinophils related with chromosome 16 abnormalities, either
inv(16)(p13q22) or chromosome 16 inversion, being associated
with a better prognosis and response to treatment [14,17,18,25].
In FC immunophenotyping, two distinct populations of leukemic
cells are typically observed based on the FSC/SSC pattern, one of
a large size (high FSC) and a smaller one (low FSC), corresponding
to blastic cells from monocyte and granulocyte lineage,
respectively [24]. Monocyte antigens CD14 and CD64 are positive
along with HLADR, CD11b, CD11c and CD36. Myeloid antigens
CD13 and CD33 are generally positive [14,17,18,25]. The AMLM4eo,
exhibits aberrant expression of the CD2 lymphoid line
antigen may occur and weak expression of the CD4 antigen can
be observed in the monocyte population [14,7-18,25].
Monocytic Leukemias (AML-M5) are defined when 80% or more
of non-erythroid cells in the BM are composed of monoblasts,
promonocytes or monocytes. The AML-M5a subtype has >80%
of monoblasts, while the LMA-M5b subtype has a predominance
of promonocytes [2-4].
The immunophenotypic profile characteristic of AML-M5 is the
presence of a leukemic population with a higher FSC/SSC ratio
than in AML subgroups M0, M1 and M2 [24]. Monocytes antigens CD14 and CD64 are positive along with HLADR, CD11b, CD11c
[14,25]. Elevated levels of CD14 expression is a specific feature
of mature monocytes, being often absent or underexpressed in
immature monocytic cells [14]. In addition, other antigens that
are generally considered to be associated with monocytes may
be expressed in other types of AML, such as CD33 and CD13
[14,25]. The CD34 is generally negative, being more present in
the most immature subgroup (AML-M5a) [1,14,17,18,25].
The CD36 is not specific and can also be seen in the erythroid,
megakarycitary and monocytic series [25]. The weak expression
of the CD4 antigen can be observed [14,25]. AML-M5 with CD33
and CD4 expression associated with negativity to CD13 and CD34
is frequently correlated with t(9;11) [1,14,25].
Patients with monocytic leukemia have a high incidence of extramedullary
disease, with infiltration in the gums, skin, digestive
tract and CNS. The presence of hepato-splenomegaly and
leukocytosis is more frequent compared to other FAB subtypes
[14]. These characteristics are consistent with the clinical,
hematological and immunophenotyping data of AML-M5a/M5b
observed in the present study (Tables 2-4).
Erythroleukemia (AML-M6) is a rare type of AL, corresponding to
2 to 3% of AML, defined by ≥ 50% of the nucleated cells in the BM
being of erythroid origin [3-4]. Two subtypes herein described
AML-M6a (erythroid/myeloid AML) with the presence of leukemic
components of erythroid and myeloid origin, and AML-M6b (pure
erythroid AML), characterized by the presence of 80% or more
of erythroid precursors in BM, and are rarely seen and clinically
more severe than AML-M6a [26]. Cytochemical reactions of MPO
and SBB are negative in leukemic erythroid cells [16].
AML-M6 can be differentiated from AM by immunophenotyping,
being made by the expression of CD235a, CD36 and strong
expression of CD71 in erythroid lineage cells [25]. Investigation of
CD33, CD117 and CD34 may also be useful, as they are negative
in MA and generally positive in erythroid cells of AML-M6. The
phenotype of the myeloid population is similar to that observed
in AML-M0/M1 [13,18,19,28,29]. Additionally, vitamin B12
dosage may be useful in diagnostic complementation, with low
or absent serum levels in MA. These characteristics of these AML
were also observed in the present study (Tables 2-4).
Transferrin receptor (CD71) is an integral membrane protein
encoded by a gene localized in chromosome 3 that mediates
cellular iron uptake by the erythroid lineage for hemoglobin
synthesis and may actively proliferate cell population, since iron
is required for cell division. It is expressed by a wide variety of
cells as erythrocyte precursors [30].
In AML, CD71 expression correlates with proliferative activity of
leukemic cells, with consensus on the co-expression of CD71 and
CD34 used to stratify AML patients as a poor prognostic factor
[30]. In present investigation, CD71 expression was found in
29.4% of all cases, being significantly higher in more immature
AML and all cases of AML-M6.
Acute Megakaryoblastic Leukemia (AML-M7) is defined by the
presence of more than 30% of megakaryoblasts among nucleated
cells [3-4]. This leukemia is rare, but relativity frequent in children
with Down syndrome [28-29].
AML-M7 can be confused with ALL or AML-M0 by morphological
criteria. Blast cells from BM are small, pleomorphic, basophilic
cytoplasm without granules, with projections (blebs) and
negative for cytochemical stains such as MPO and SBB [3-4].
The main immunophenotypic characteristic of blastic cells from
AML-M7 is the expression of platelet antigens CD41 (glycoprotein
complex llb/llla), CD42 (glycoprotein lb) and CD61 (glycoprotein
llla). The expression of pan-myeloid antigens CD13 and CD33 is
described, as has positive cases of CD36. Some cases can be HLADR
and CD34 negative [13,8-19,26]. These immunophenotyping of
AML-M7 characteristics were also observed in the present study
(Table 4).
In addition to the distinction between AML and ALL and AL
classification, immunophenotyping allowed the identification
of additional prognostic factors, allowing a better stratification
into risk groups, enabling a differentiated therapeutic approach.
Positivity to CD34, CD71, aberrant expression of lymphoid
antigens and Multidrug Resistance (MDR) phenotype are
highlighted as factors of poor prognosis of AML [1,8,19,25,27,30- 32].
CD34 is a 105-120 KD glycoprotein expressed on many different
cell types, more specifically in immature hematopoietic stem
cells mediating the binding of hematopoietic stem cells to
extracellular matrix proteins or stromal cells in the precursors
B and T lymphocytes and even more immature Colony-Forming
Cells (CFUs) and myeloblastic [31].
In AML, CD34 has in 45-68% of cases with a higher incidence in
more immature subtypes us M0, M1 and M5a, and this expression
of has adverse prognostic factors, such as a higher recurrence
rate and refractory disease [6,31].
Some authors have reported that the prognostic significance of
CD34 expression increases if co-expression occurs with other
antigens such as HLADR [6], CD71 [30] and aberrant expression of
CD7 [31-36]. A high intensity of co-expression of CD34 together
with CD19 and CD56 is characteristic of AML-M2 with t(8;21)
translocation [17,18].
It has also been reported that among AML patients co-expressing
CD34 together with MDR proteins such us P-glycoprotein (Pgp)
and multidrug resistance-associated protein-1 (MRP1) they was
a statistically significant lower rate of complete remission or
shorter overall survival [19].
In present study, we found CD34 positivity in 106 cases of AML,
with more expressive levels in AML with very immature blasts
(M0, M1 and M5a), in contrast to groups M2, M5b, which showed
lower levels of positivity and negativity in all cases of AML-M3
(Table 4).
Aberrant phenotypes are associated with AL, identified by
the co-expression of cell markers that are rarely or never
found simultaneously in normal hematopoietic differentiation,
overexpression of a specific cell line marker or absence of a
marker, which configures the maturation asynchronism of a cell
line [1,18,19,25,36-38].
Some immunological and molecular studies have reported that
many AL may present antigenic characteristics of more than one cell line, characterizing two groups of leukemias that presented
“lineage infidelity”, that is, ALL expressing antigens associated
with the myeloid lineage and AML expressing antigens of
lymphoid lineage [1,8,13,36-38].
However, it is important to make a distinction between
biphenotypic leukemia with ALL or AML with aberrant expression
of markers from other strains, due to differences in therapeutic
approaches, therefore it is recommended to investigate the
scoring system suggested by the EGIL group [13], as well as
the use of a panel made up of multiple MoAb combined with
different fluorochromes, which makes it possible to investigate
the expression of different antigens in the same cell by means of
FC [17,18].
The mechanisms by which the expression of aberrant phenotypes
occurs in the development of LAs remain unclear, however,
it is possible to establish associations between these unusual
expressions and other biological characteristics of the disease,
such as associations with chromosomal translocations and
adverse prognostic factors [17,18,25,30,32-36].
According to the WHO, several lymphoid immunophenotypic cell
markers may be aberrantly expressed in AML [25,26]. TdT may be
expressed in greater than one-third of cases, CD7, CD2, CD19 and
CD56 may be expressed frequently; however, the T-cell antigen
CD3 is usually absent [1,18,19,25].
TdT is a nuclear polymerase normally expressed during the early
stages of B and T cell differentiation [37]. In initial reports, TdT
expression was believed to be limited to ALL and can account
in 18-24% of AML, more frequently in subtype M0 and M1. No
specific chromosomal abnormalities were associated with AML
TdT+ 37.
CD7 is a 40kDa glycoprotein encoded by a gene situated on
chromosome 17 [33]. In T-cells CD7 play an important role in
the cellular activation. Some authors, however, believe that the
expression of this antigen in T progenitor cells could be related
to mediating the migration of these cells from the MB to the
thymus. It is identified in hematopoietic progenitor cells that can
give rise to other cell lines and can thus be observed in AML more
frequently in subtypes M0 and M1 [33].
According to some authors, the co-expression of CD7 with other
cellular markers related to poor prognosis in AMLs such as
CD34, Pgp and MRP1 [19]. In our investigation, CD7 expression
was observed in 32/143 patients, most of whom had CD34 coexpression,
characterized AML very immature (Table 4).
CD2 is a 45-58kD glycoprotein present on the surface of T
lymphocytes and natural-killer cells (NK) and is not normally
expressed in human myeloid cells, but is found in a significant
minority of AML cases, M2, M3v, M4 and M5 groups
[17,18,25,36]. Expression of CD2 and other T-lymphoid antigens
such as CD4, CD7 and CD56 in myeloid blast cells are correlated
with extramedullary disease [17,18,25,36].
CD19 is a 95kD glycoprotein, which appears very early during
the maturation of B-lymphocyte precursors and is constitutively
expressed in mature normal B-lymphocytes and related
neoplasm but not in plasma cells [32,35]. CD19 expression has been observed in 2-22% of AML cases commonly associated with
AML-M2 with translocation t(8;21) and t(8;19) and also in AML
subgroups M3v, M4 and M5 [17,18,25]. In the present study,
CD19 and CD2 expression were observed in 8.4% and 6.7% of
cases respectively, with a predominance of CD19 in M2 and CD2
in M3 subgroup of AML (Table 3).
Neural cell adhesion molecule (NCAM or CD56) is a 180 kD
glycoprotein, encoded by a gene located in chromosome 11, and
it is expressed on most normal NK cells [38]. In addition, CD56
expression was found in rare subsets of T-lymphocytes (NKT
cell), dendritic cells, and neural and mesenquimal stem cells [8].
Aberrant expression of CD56 in AML is present in 13-29% of cases
with high frequency in AML M2, M3 and M5. It identifies a subset
of patients with a bad prognosis as extramedullary involvement
and high leukocyte count in the AML-M2 with t(8;21) and t(15;17)
[38-40]. In our samples, CD56 expression was observed in 23
cases with predominance in AML-M2 cases (Table 4).
Conclusion
We conclude that the immunophenotypic patterns observed in
patients with AML allowed the accurate identification of different
groups of this leukemia. Additionally, through CF we also identify
varied patterns of aberrant phenotypes that also contribute to
the diagnosis and identification of prognostic factors.
Author Contributions
Linduarte V. Morais, Taissa Maria M. Oliveira, Erica A. Gil, Lenilton
S Silveira Jr, Victor L Soares and Rafael L Duarte, collected the data
and contributed to the writing of the manuscript. Dany Kramer,
Aldair S Paiva and Gustavo Oliveira contributed to the clinical
interpretation of laboratory analysis. Geraldo B. Cavalcanti Jr
conceived and conducted the study, contributed clinical and flow
cytometry data, and reviewed the manuscript.
Conflict of Interest
The authors declare that there is no conflict of interest of any
kind.
REFERENCES
- Döhner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, et al. (2010) Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the diagnosis and management of acute myeloid leukemia in adults. On behalf of the European LeukemiaNet. Blood 115: 453-474.
Indexed at, Google Scholar, Cross Ref
- Zini G (2021) How I investigate difficult cells at the optical microscope. Int J Lab Hematol 43: 346-353.
Indexed at, Google Scholar, Cross Ref
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DAG, et al. (1976) Proposal for the Classification of the acute leukemias. Brit J Haematol 33: 451-488.
Google Scholar, Cross Ref
- Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DAG et al. (1985) Proposed revised criteria for the classification of acute myeloid leukemia. Ann Int Med 103: 620-625.
Indexed at, Google Scholar, Cross Ref
- Casasnovas RO, Slimane FK, Garand R, Faure GC, Campos L, et al. (2003) Immunological classification of acute myeloblastic leukemias: relevance to patient outcome. Leukemia 17: 515-517.
Indexed at, Google Scholar, Cross Ref
- Tong H, Lu C, Zhang J, Liu Z, Ma Y, et al. (2009) Immunophenotypic, cytogenetic and clinical features of 192 AML patients in China. Clin Exp Med 9: 149-155.
Indexed at, Google Scholar, Cross Ref
- Gajendra S (2016) Flow cytometry in acute leukemia. Clin Oncol 1: 1-4.
Indexed at, Google Scholar, Cross Ref
- Haycocks NG, LawrenceL, Cain JW, Zhao F (2011) Optimizing antibody panels for efficient and cost-effective flow cytometric diagnosis of acute leukemia. Cytometry B (Clin Cyt) 80: 221-229.
Indexed at, Google Scholar, Cross Ref
- Weir EG, Borowitz MJ (2001) Flow cytometry in the diagnosis of acute leukemia. Sem Hematol 38: 124-138.
Indexed at, Google Scholar, Cross Ref
- Campana D, Behm FG (2000) Immunophenotyping of leukemia. J Immunol Meth 243: 59-75.
Google Scholar, Cross Ref
- Bain BJ, Barnett D, Linch D, Matutes E, Reilly JT, et al. (2002) Revised guideline on immunophenotyping in acute leukaemias and chronic lymphoproliferative disorders. Clin Lab Haematol 24: 1-13.
Indexed at, Google Scholar, Cross Ref
- Bene MC, Castoldi G, Knapp W, Matutes E, Orfao A, et al. (1995) Proposals for the immunological classification of acute leukemias: European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 9: 1783-1786.
Indexed at, Google Scholar
- Kaleem Z, Crawford E, Pathan MH, Jasper L, Covinsky MA, et al. (2003) Flow cytometric analysis of acute leukemias: diagnostic utility and critical analysis of data. Arch Pathol Lab Med 127: 42-48.
Indexed at, Google Scholar, Cross Ref
- Xu Y, McKenna, R, Wilson, K. Karandikar NJ, Schultz RA, et al. (2006) Immunophenotypic identification of acute myeloid leukemia with monocytic differentiation. Leukemia 20: 1321–1324.
Indexed at, Google Scholar, Cross Ref
- Douer D (2003) The epidemiology of acute promyelocytic leukaemia. Best Pract Res Clin Haematol 16: 357-367.
Indexed at, Google Scholar, Cross Ref
- Bain BJ, Swirsky (2012) Erythrocyte and leucocyte cytochemistry in Bain BJ, Bates I, Laffan MA, Lewis SM. Dacie and Lewis Practical Haematology. Chapter 15, (11th Edition) Elsevier Churchill Livingstone, London. 333-352.
- Ikoma MR, Sandes AF, Thiago LS, Cavalcanti GB, Lorand-Metze IGH, et al. (2015) First Proposed panels on acute leukemia for four-color immunophenotyping by flow cytometry from the Brazilian Group of Flow Cytometry-GBCFLUX. Cytometry B (Clin Cyt) 88: 194-203.
Indexed at, Google Scholar, Cross Ref
- Beltrame MP, Xisto ES, Yamamoto M, Furtado FM, da Costa ES, et al. (2021) Updating recommendations of the Brazilian Group of Flow Cytometry (GBCFLUX) for diagnosis of acute leukemias using four-color flow cytometry panels. Hematol Transfus Cell Ther 43: 1-8.
Indexed at, Google Scholar, Cross Ref
- Silveira Júnior LS, Lima Soares V, Jardim da Silva AS, Aires Gil E, Pereira de Araújo MG, et al. (2020) P-glycoprotein and multidrug resistance-associated protein-1 expression in acute myeloid leukemia: Biological and prognosis implications. Int J Lab Hematol 42: 493-668.
Indexed at, Google Scholar, Cross Ref
- Alves GV, Fernandes AL, Freire JM, Paiva AS, Vasconcelos RC, et al. (2012) Flow cytometry immunophenotyping evaluation in acute lymphoblastic leukemia: Correlation to factors affecting clinic outcome. J Clin Lab Anal 26: 431-440.
Indexed at, Google Scholar, Cross Ref
- Cavalcanti Júnior GB, Vasconcelos FC, Faria GP, Scheiner MAM, Dobbin JA, et al. (2004) Coexpression of p53 protein and MDR functional phenotype in leukemias: The predominant association in chronic myeloid leukemia. Cytometry B (Clin Cyt) 61: 1-8.
Indexed at, Google Scholar, Cross Ref
- Cavalcanti Júnior GB, Scheiner MAM, Magluta EPS, Vasconcelos FC, Klumb CE, et al. (2010) P53 flow cytometry evaluation in leukemias: correlation to factors affecting clinic outcome. Cytometry B (Clin Cyt) 78: 253-259.
Indexed at, Google Scholar, Cross Ref
- Vidriales MB, Orfao A, Lópes-Berges MC, González M, López-Macedo, et al. (1995) Light scatter characteristics of blasts cells in acute myeloid leukaemia: association with morphology and immunophenotype. J Clin Pathol 48: 456-462.
Indexed at, Google Scholar, Cross Ref
- BJ Bain, MC Béné (2019) Morphological and immunophenotypic clues to the WHO categories of acute myeloid leukaemia. Acta Haematol 141: 232-244.
Indexed at, Google Scholar, Cross Ref
- Szczepanski T, van Velden HHJ, van Dongen JJM (2003) Classification systems for acute and chronic leukemias. Best Pract Res Clin Hamatol 16: 561-582.
Google Scholar, Cross Ref
- Ortoloni C (2011) Flow cytometry of hematological malignancies. Chapter 1- Antigens: Myeloperoxidase. Page 100. Willey-Blackwell. A John Willey & Sons Ltd.
- Pande A, Dorwal P, Jain D, Tyagi N, Mehra S, et al. (2016) Expression of CD71 by flow cytometry in acute leukemias: More often seen in acute myeloid leukemia. Ind J Pathol Microbiol 59: 310-313.
Indexed at, Google Scholar, Cross Ref
- Athale UH, Razzouk BI, Raimondi SC, Tong X, Behm FG, et al. (2001) Biology and outcome of childhood acute megacaryoblastic leukemia: a single institution’s experience. Blood 97: 3727-3732.
Indexed at, Google Scholar, Cross Ref
- Pagano L, Pulsoni A, Vignetti M, Mele L, Fianchi L, et al. (2002) Acute megakaryoblastic leukemia: experience of GIMEMA trials. Leukemia 16: 1622-1628.
Indexed at, Google Scholar, Cross Ref
- Li X, Li J, Du W, Zhang J, Liu W, et al. (2010) Relevance of immunophenotypes to prognostic subgroups of age, WBC, platelet count, and cytogenetics in de novo acute myeloid leukemia. APMIS 119: 76-84.
Indexed at, Google Scholar, Cross Ref
- Ortoloni C (2011) in Flow cytometry of hematological malignancies. Chapter 1- Antigens: CD34 antigen. Page 66-68. Willey-Blackwell. A John Willey & Sons Ltd.
- Ossenkoppele GJ, van de Loosdrecht AA, Schuurhuis GJ (2011) Review of the relevance of aberrant antigen expression by flow cytometry in myeloid neoplasms. Brit J Haematol 153: 421-436.
Indexed at, Google Scholar, Cross Ref
- Chang H, Yeung J, Brandwein J, Qi-long Y (2007) CD7 expression predicts poor disease-free survival and post-remission survival in patients with acute myeloid leukemia and normal karyotype. Leuk Res 31 (2), 157-162.
Indexed at, Google Scholar, Cross Ref
- Tiftik N, Bolaman Z, Batun S, Ayyildiz O, Isikdogan A, et al. (2004) The importance of CD7 and CD56 antigens in acute leukemias. Int J Clin Pract 58: 149-152.
Indexed at, Google Scholar, Cross Ref
- Mushsin S, Al-Mudallal S (2014) Expression of aberrant antigens CD7 and CD19 in adult acute myeloid leukaemia by flow cytometry. Iraqi J Hematol 3: 1-13.
Google Scholar
- Shahni A, Saud A, Siddiqui S, Murky SN (2018) Expression of aberrant antigens in hematological malignancies: A single center experience. Pak J Med Sci 34: 457-462.
Indexed at, Google Scholar, Cross Ref
- Ortoloni C (2011) Flow cytometry of hematological malignancies. Chapter 1- Antigens: Terminal Deoxynucleotidil Transferase. Willey-Blackwell. A John Willey & Sons Ltd.
Google Scholar
- Sasca D, Sybink J, Schuler A, Shah V, Heidelberger J, et al. (2019) NCAM1 (CD56) promotes leukemogenesis and confers drug resistance in AML. Blood 133: 2305-2319.
Indexed at, Google Scholar, Cross Ref
- Raspandori D, Damiani D, Lenoci, Rondelli D, Testoni N, et al. (2001) CD56 expression in acute myeloid leukemia identifies patients with poor clinical prognosis. Leukemia 15 1161-1164.
Google Scholar
- Baer MR, Stewart CC, Lawrence D, Arthur DC, Byrd JC, et al. (1997) Expression of the neural cell adhesion molecule CD56 is associated with short remission duration and survival in acute myeloid leukemia with t(8; 21)(q22; q22). Blood 90: 1643-1648.
Indexed at, Google Scholar
Citation: Morais LV, Lima RD, Gil EA, Paiva AS, Junior LSS, et al. (2022) Clinical Significance of Flow Cytometry Findings in Brazilian Patients with De Novo Acute Myeloid Leukemia. Ann Clin Lab Res. Vol.10 No.6:417







