Introduction
Toxocariasis is one of the most commonly reported zoonotic helminthes infection in the world recorded in both developing countries; where it is usually associated with low socioeconomic level [1], and developed countries, especially in rural areas among children who have pets [2,3]. Toxocariasis seroprevalence has been reported to fluctuate between 2 and 92.8% according to geographical region and age group [4,5].
Human infection occurs by accidental ingestion of embryonated eggs of Toxocara canis or Toxocara cati, the intestinal parasites of dogs and cats, respectively [6] where only few larvae are needed to cause disease [7]. Contact with contaminated soil is considered to be the primary route of transmission of Toxocara in humans [8], in addition to, direct contact with dog carrying eggs in their fur [9]. Other methods of transmission include: geophagia, poor personal hygiene as well as consumption of raw vegetables grown in contaminated gardens [10].
Migration of infected larvae through the human tissues, in the majority of cases does not result in clinical manifestation [11]. However, in parasitized individuals with the presence of anti-Toxocara antibodies visceral larva migrans is induced (VLM), which affects mainly young children and characterized by leukocytosis, fever, hepatomegaly, hypergammaglobulinemia, coughing, wheezing, abdominal pain, and elevated serum IgE level [12,13]. Eosinophilia is one of the most outstanding characteristic of the VLM in naturally infected humans and experimental models of infection [3,8].
Human toxocariasis is still a poorly diagnosed disease hence largely unknown either to health professionals and/or the general of Egyptian population [6]. It’s difficult to be diagnosed by the direct techniques thus ELISA is used most often in diagnosis and during epidemiologic studies. ELISA has high sensitivity and specificity with insignificant levels of cross-reactivity reported when the excretory and secretory antigens of the T. canis larvae second-stage are used [14,15].
Asthma is considered the most common chronic disease of childhood [16]. Atopy is an important risk factor for asthma, since about half of asthma cases are atopic individuals [17] as refereed by the collected epidemiological records which suggested that Toxocara infection had contributed significantly in the pathogenesis of atopy [18]. Most previous studies revealed no association between toxocariasis and asthma [19] but positive correlation was proved by other studies [20,21].
Due to these controversy results, the present study aimed to investigate the Toxocara seropositivity in asthmatic children, in our locality (Mansoura city, Egypt) and to record its association with clinical signs, laboratory tests and epidemiological factors.
Material and Methods
Sampled participants
The study was carried out on asthmatic children attending Allergy and Immunology outpatient clinic at Mansoura University Children hospitals, Egypt during the period from January 2010 to January 2012. The study protocol was approved by the institutional committee for the protection of human subjects and the Ethics Committee of Faculty of medicine, Mansoura University. Asthma cases were diagnosed according to the GINA recommendations [17]. The controls were attendee of other outpatient clinics with no respiratory complaint. Their age range was from 2 to 15 years. A questionnaire forms were prepared and given to parents of each case within both groups. The questionnaire contained epidemiological data including: age, sex, address (either urban or rural), geophagia and contact with pets, were recorded in a preplanned sheet format and then the child was subjected to full physical examination. It worth to be mentioned that the exclusion criteria among the studied population was for all cases positive for nematodes infection by stool examination (eg, ascariasis, enterobiasis, strongyloidiasis and trichostrongyliasis) to overcome cross reactivity with toxocariasis.
Sample collection
Five ml of venous blood was collected from each child placed in 2 tubes, one containing anticoagulant for CBC with differential leukocytes count. Especially looking for those with Eosinophilia corresponded to levels above 400/ mm3 [3]. The blood sample in the second tube is centrifuged then collected serum was divided into two tubes, one for Toxocara serology and the other for measurements of total IgE. Serum samples were stored at −20°C until used. Stool samples were also obtained from all enrolled children and examined. Positive cases for other nematode infection were excluded to eliminate possible cross-reactions.
Serological investigation
Serum samples were tested for both anti-Toxocara IgG and IgE antibodies. The IgG detection was done using RIDASCREEN Toxocara-IgG ELISA kit (R-Biopharm AG, Germany) which detect antibodies against the excretory/secretory antigen of the T. canis larvae. While quantitative determination of total IgE antibodies concentration was carried out by IgE ELISA kit (Immunospec corporation, CA), where levels above 200 IU/ml were considered high. In both procedures, the manufacturer’s guidelines were followed. Before testing for anti-Toxocara IgG antibodies serum samples were preincubated with Ascaris antigen to avoid immunologic crossreactivity between Toxocara and Ascaris.
Statistical analysis
All data were introduced in an Excel spreadsheet and statistical analysis was carried out using the SPSS statistical package version 16 (SPSS, Inc., Chicago, IL, USA). Chi-square test and Odds Ratio (OR) were performed to confirm the difference between groups. The level of significance selected was p value < 0.05.
Results
The total of 120 asthmatic children besides 60 non-asthmatic controls, were included in the study. The age (mean±SD) of asthmatic group and control one were (5.73±3.493) and (6.01±3.048), respectively, with no significant difference between both groups (P>0.05). Regarding the clinical and epidemiological factors, there was significant difference between asthmatic group and control group regarding geophagia, splenomegaly and hepatomegaly (P<0.05) but as regard gender, locality, lymphadenopathy and contact with pets there was no difference between both groups (P>0.05), as shown in (Table 1). Concerning the laboratory investigations, significant difference was detected between asthmatic and control group regarding IgE level, anti- Toxocara IgG and eosinophilia (Table 2).
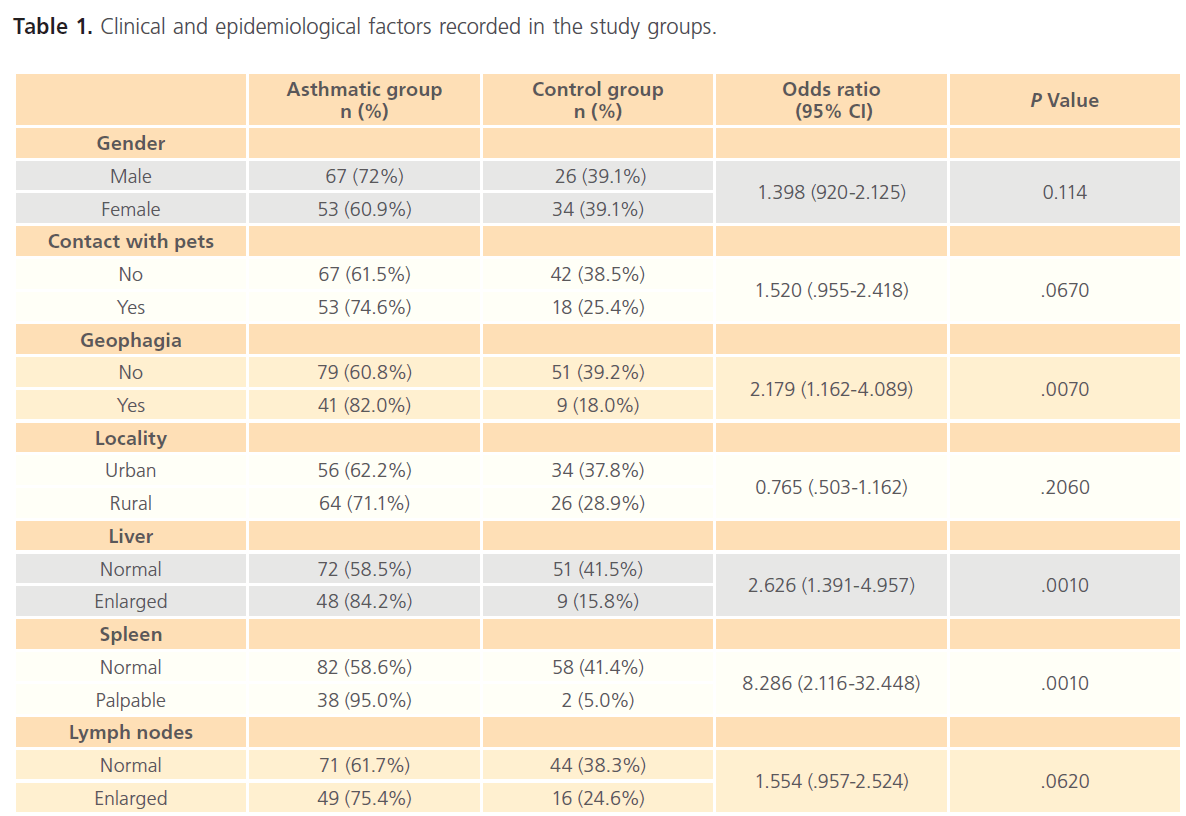
Table 1: Clinical and epidemiological factors recorded in the study groups.
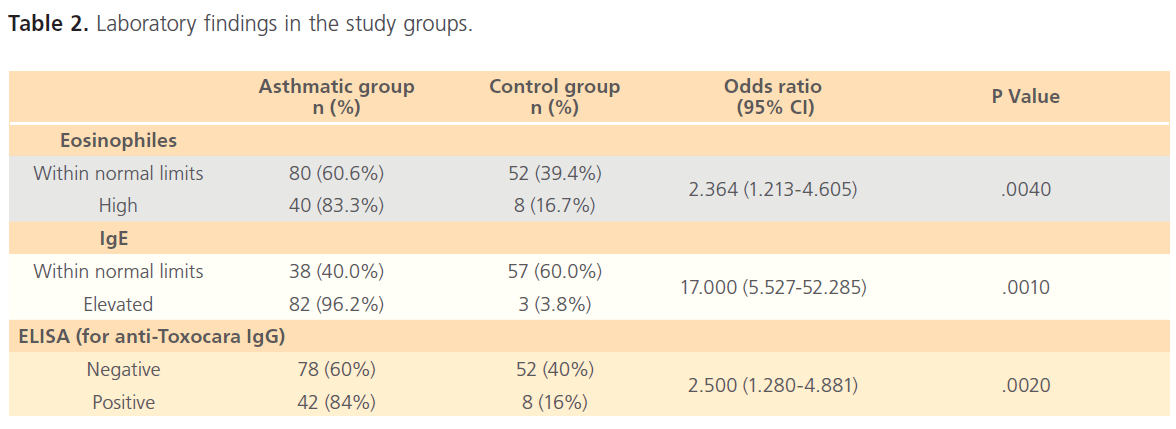
Table 2: Laboratory findings in the study groups.
DiscussionAsthma has increased in industrialized countries recently [22]. Geohelminthes infection is considered as a potential environmental risk factor for asthma [23,24]. Reactive respiratory changes occur in VLM, caused by Toxocara infection, because of larval migration and infected individuals are more likely to wheeze in response to larval invasion [13]. Studies on the association between Toxocara seropositivity and asthma have provided conflicting evidence with some showing positive association in human [20,21] and in animal model [25], while others shown no relation [19]. This casecontrol study aimed to investigate Toxocara seropositivity in our locality (Mansoura city, Egypt), as a developing country, among asthmatic children as they are more prone to infection.
Our result demonstrated a positive association between asthma and Toxocara seropositivity which was concordant with cross-sectional study of children carried out in The Netherlands that demonstrated occurrences of asthma/recurrent bronchitis significantly associated with Toxocara seroprevalence [20]. Ferreira et al. (2007) carried their study among under-five Amazonian children demonstrated that 21.5% of examined children had antibodies to Toxocara and the increased risk of asthma was independently associated with seropositivity to Toxocara [21]. Our results agree with previous observations of higher Toxocara seropositivity rates in asthmatic children [26]. Contrarily, Sharghi et al. [19] didn’t find any association between Toxocara seropositivity and asthma among 95 asthmatic and 229 non asthmatic children which was attributed to the lower number of asthmatic cases in that study.
There is a significant relation between Toxocara infection and geophagia in the present study. Previous studies reported that geophagia is an important risk for Toxocara infection [20,27]. In our locality, infection can occur in both urban and rural areas due to contact with Toxocara ova contaminating ground from stray infected dogs or contact with domestic pets, respectively.
We have observed that the reported clinical signs suggestive of toxocariasis have a correlation with positive Toxocara serology. This is in accordance with the previous results of Teixeira et al. [28] who founded that children with seroprevalence for Toxocara have a significant relation to enlarged liver and spleen (p= .0010). Other studies reported that toxocariasis should be considered in differential diagnosis of eosinophilia, in patients with bronchospasms, isolated hepatomegaly and splenomegaly [29]. It was stated that T. canis infection must be considered in at-risk children, such as those with puppies at home, who have had contact with soil, who have hepatomegaly and/or asthma with eosinophilia and increased serum IgE [30]. Bahnea et al., reported that the most frequent clinical manifestation was pulmonary symptoms and the most frequent digestive symptoms were abdominal pain and hepatosplenomegaly in adult with positive anti-Toxocara antidodies. The laboratory diagnosis of their study population revealed, hypereosinophilia in 94.73% childrens associated with hyperleucocytosis and hyper-gamma globulinemia. All the patients were serologically confirmed with toxocariasis [31].
In the present work, a statistical significance between the asthmatic group and the control group regarding eosinophilia, IgE and anti –Toxocara IgG (p= 0.004, 0.001, 0.002 respectively) was detected. These results are in accordance with other studies suggesting that eosinophilia and increased serum IgE in asthmatic patients are highly suggestive of toxocariasis [29-31].
In conclusion, this study demonstrated a significant correlation between Toxocara seropositivity and bronchial asthma in pediatric asthmatic population in our locality emphasizing the role concerning Toxocara infection as an important environmental risk factor for asthma. It’s important to high light this neglected parasite as a potential risk factor for asthma. Physicians should pay attention to incorporate toxocariasis in the differential diagnosis of asthma with hepatomegaly and splenomegaly.
Acknowledgment
Special thanks are due to Prof. Atef El-Shazly (Parasitology department, Mansoura University, Egypt) and Prof. Magdy Zedan (Allergy Unit, Mansoura University, Egypt) for their encouragement and advice in this study.
98
References
- Hotez, PJ. Visceral and ocular larva migrans. Semin Neurol. 1993; 13: 175-179.
- Dubinský, P., Havasiová-Reiterová, K., Petko, B., Hovorka, I., Tomasovicová, O. Role of small mammals in the epidemiology of toxocariasis. Parasitology 1995; 2: 187-193.
- Figueiredo, SD., Taddei, JA., Menezes, JJ., Novo, NF., Silva, EO. et al. Clinical-epidemiological study of toxocariasis in a pediatric population. J Pediatr. 2005; 81: 126-132.
- Magnaval, JF., Michault, A., Calon, N., Charlet, JP., De Larrard, B. Epidemiology of human toxocariasis in La Reunion. Trans R Soc Trop Med Hyg. 1994; 88: 531-533.
- Park, HY., Lee, SU., Huh, S., Kong, Y., Magnaval, JF. A seroepidemiological survey for toxocariasis in apparently healthy residents in Gangwon-do, Korea. Korean J Parasitol. 2002; 40: 113-117.
- Despommier, D. Toxocariasis: clinical aspects, epidemiology, medical ecology, and molecular aspects. Clin Microbiol Rev. 2003; 16: 265-272.
- Taylor, MR., O’Connor, P., Keane, CT., Mulvihill, E., Holland, C. The expanded spectrum of toxocaral disease. Lancet 1988; 26: 692-658.
- Paludo, LM., Falavigna, DLM., Rubinski-Elefant, G., Gomes, LG., Baggio, MLM. et al. Frequency of Toxocara infection in children attended by the health public service of Maringá, South Brazil. Rev Inst Med Trop Sao Paulo 2007; 49: 343-348.
- Roddie, G., Holland, C., Stafford, P., Wolfe, A. Contamination of fox hair with eggs of Toxocara canis. J Helminthol. 2008; 82 (4): 293-296.
- Mizgajska-Wiktor, H., Uga, S. Exposure and environmental contamination. Holland, CV., Smith, HV. eds. Toxocara: The Enigmatic Parasite, CABI International. 2006. pp. 211-227.
- Magnaval, JF., Glickman, LT., Dorchies, P., Morassin, B. Highlights of Human Toxocariasis. Korean J Parasitol. 2001; 39: 1-11.
- Alderete, JM., Jacob, CM., Pastorino, AC., Elefant, GR., Castro, AP. et al. Prevalence of Toxocara infection in school children from the Butantã region, São Paulo, Brazil. Mem Inst Oswaldo Cruz 2003; 98: 593-597.
- Nash, TE. Visceral larva migrans and other unusual helminth infections. Mandell, GR., Bennett, JE., Dolin, R. eds. Principles and Practice of Infectious Diseases, Philadelphia: Elsevier Churchill Livingstone. 2005.
- Magnaval, JF., Fabre, R., Maurières, P., Charlet, JP., De Larrard, B. Evaluation of an immunoenzymatic assay detecting specific anti-Toxocara immunoglobulin E for diagnosis and post-treatment follow-up of human toxocariasis. J Clin Microbiol. 1992; 30: 2269-2274.
- Garcia, LS. Tissue Nematodes: Diagnostic Medical Parasitology. Washington DC: ASM press. 2001. p. 309.
- Halfon, N., Newacheck, PW. Childhood asthma and poverty: differential impacts and utilization of health services. Pediatrics 1993; 91: 56-61.
- GINA. Global Strategy for Asthma Management and Prevention. NHLBI/WHO workshop report, revised. National Institute of Health/ National Heart, Lung and Blood Institute. NIH publication No. 02-5639. 2002.
- Chan, PW., Anuar, KA., Fong, M., Debruyne, JA., Ibrahim, J. Toxocara seroprevalence and childhood asthma among Malaysian children. Pediatr Intern. 2001; 43: 350-353.
- Sharghi, N., Schantz, PM., Caramico, L., Ballas, K., Teague, BA. et al. Environmental exposure to Toxocara as a possible risk factor for asthma: A clinic-based case-control study. Clin Infect Dis. 2001; 32: 111-116.
- Buijs, J., Borsboom, G., van Gemund, JJ., Hazebroek, A., van Dongen, PA. Toxocara seroprevalence in 5-year-old elementary schoolchildren: Relation with allergic asthma. Am J Epidemiol. 1994; 140: 839-847.
- Ferreira, MU., Rubinsky-Elefant, G., de Castro, TG., Hoffmann, EH., da Silva-Nunes, M. et al. Bottle feeding and exposure to Toxocara as risk factors for wheezing illness among under-five Amazonian children: a population based cross-sectional study. J Trop Pediatr. 2007; 53: 119-124.
- Eder, W., Ege, MJ., von Mutius, E. The asthma epidemic. N Engl J Med. 2006; 355: 2226-2235.
- Cooper, PJ. Intestinal worms and human allergy. Parasite Immunol. 2004; 26: 455-467.
- van Riet, E., Hartgers, FC., Yazdanbakhsh, M. Chronic helminth infections induce immunomodulation: consequences and mechanisms. Immunobiology 2007; 212: 475-490.
- Pinelli, E., Brandes, S., Dormans, J., Gremmer, E., van Loveren, H. Infection with the roundworm Toxocara canis leads to exacerbation of experimental allergic airway inflammation. Clin Exp Allerg. 2008; 38: 649-658.
- Kanobana, K., Vereecken, K., Junco Diaz, R., Sariego, I., Rojas, L. et al. Toxocara seropositivity, atopy and asthma: a study in Cuban schoolchildren. Trop Med Int Health. 2013; 18: 403-406.
- Teixeira, CR., Chieffi, PP., Lescano, SAZ., Silva, EOM., Fux, B., Cury, MC. Frequency and risk factors for toxocariasis in children from a pediatric outpatient center in southeastern Brazil. Rev Inst Med Trop Sao Paulo 2006; 48: 251-255.
- Saporito, L., Scarlata, F., Colomba, C., Infurnari, L., Giordano, S. et al. Human toxocariasis: a report of nine cases. Acta Paediatr. 2008; 97: 1301-1302.
- Figueiredo, SD., Taddei, JA., Menezes, JJ., Novo, NF., Silva, EO. et al. Clinical-epidemiological study of toxocariasis in a pediatric population. J Pediatr. 2005; 81: 126-132.
- Bahnea, RG., Ivan, A., Cârdei, E., Luca, MC., Stoica, O. Retrospective clinical and laboratory study of the toxocariasis cases hospitalised between 2005 and 2008. Rev Med Chir Soc Med Nat Iasi 2008; 112: 938-941.







