Background:  Several congenital malformations such as craniofacial and digital abnormalities, and less commonly, cleft lip and palate have been reported with phenobarbital. According to our knowledge, it is the first report of cloacal anomaly and cleft palate following prenatal phenobarbital exposure.
Case: A 20-year-old gravid 1, Para 1 woman at 40 weeks of gestation was admitted for a normal vaginal delivery. She had a history of convulsion one month before pregnancy. The described dosage of Phenobarbital by her physician was 100mg every night but she had increased the dosage to 100mg q8h incidentally. Other medication was folic acid in usual dosage and continued until delivery. There was no familial history of birth defects, any antenatal infection or exposure to any other medications, alcohol, smoking, or exposure to X-rays. Pregnancy was uncomplicated and a baby girl weighing 2600g was born. APGAR scores were 8 and 9 at 1 and 5 minutes, respectively. By clinical picture, diagnosis of cloacal anomaly was confirmed. The other anomalies were cleft palate, a small patent ductus arteriosus in echocardiography and a short sacrum. A nuclear renal scan, which was performedafter two months, suggested severe decrease perfusion and function of right kidney. The patient was treated with multiple operations on oral, urogenital and anorectal system.Â
Conclusion: In addition to previously recognized risks associated with phenobarbital during pregnancy, cloacal anomaly should be considered as a possible adverse outcome.
Introduction
Phenobarbital is the oldest antiepileptic drug (AED) currently in use. Several congenital malformation such as craniofacial and digital abnormalities, and less commonly, cleft lip and palate have been reported with AEDs including Phenobarbital [1,2,3]. In this report, we present a patient who received Phenobarbital more than the recommended dose and her offspring was born with two major malformations including cloacal anomaly and cleft palate. As well as we know, this is the first report of cloacal anomaly with Phenobarbital.
Case Report
A 20-year-old gravid 1, Para 1 woman at 40 weeks of gestation was admitted for a normal vaginal delivery. She had a history of convulsion one month before pregnancy for which she was treated with Phenobarbital 100mg q8h in the first trimester of pregnancy. The described dosage of Phenobarbital by her physician was 100mg every night but she had increased the dosage to 100mg q8h incidentally. Other medication was folic acid in usual dosage and continued until delivery. The father was 25-years old and there was no consanguinity. There was no familial history of birth defects, any antenatal infection or exposure to any other medications, alcohol, smoking, or exposure to X-rays. The result of prenatal ultrasound was moderate polyhydramnius and a baby weighing 2600g was born. The karyotype of the offspring was female. APGAR scores were 8 and 9 at 1 and 5 minutes, respectively. By clinical picture diagnosis of cloacal anomaly (figure 1) was confirmed. (Table 1).
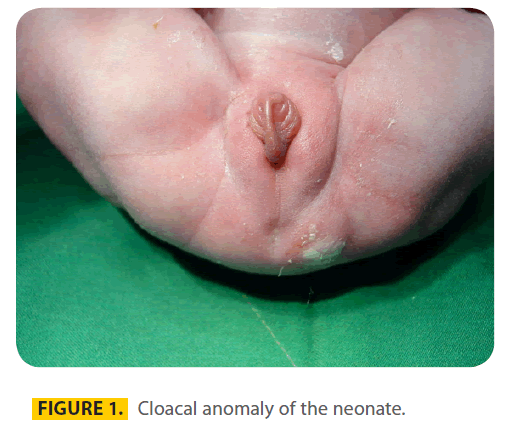
Figure 1: Cloacal anomaly of the neonate.

Table 1: Clinical findings of patient.
The other anomalies were cleft palate (hard and soft) and a small patent ductus arteriosus in echocardiography. X-ray (figure 2) showed obviously a short sacrum.
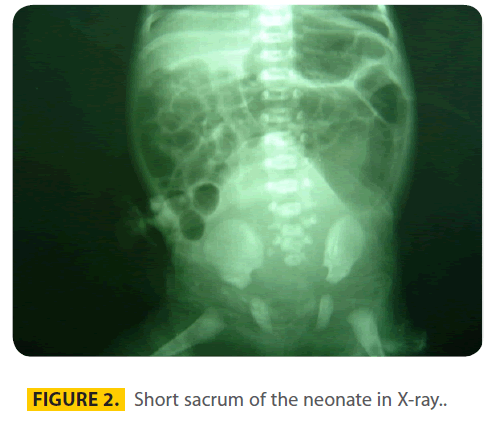
Figure 2: Short sacrum of the neonate in X-ray..
A voiding cystouretrography showed right vesicoureteral reflux (grade four). A nuclear renal scan, which was performed after two months, suggested severe decrease perfusion and function of right kidney. The patient was treated with multiple operations on oral, urogenital and anorectal system.
Discussion
It has long been recognized that AEDs increase the risk of malformation in fetus. The most common major malformations associated with AED exposure include cardiac (e.g., ventricular septal defect), orofacial (e.g., cleft lip with or without cleft palate), urological (e.g., hypospadias), skeletal (e.g., radial ray defects, phalangeal hypoplasia), and neural tube defects (NTDs) [2]. Congenital malformations including cleft lip and palate are not new adverse pregnancy outcomes with phenobarbital as they have been reported in the several reports and registries [3,4,5,6].
In the North American Registry [3], phenobarbital was associated with 6.5% of major congenital abnormalities with a relative risk (RR) of 2 versus other AEDs. Similar findings was reported by Tanaka et al [3] as phenobarbital use was associated with retarded fetal growth and 6.7% of congenital malformation that was higher than carbamazepine (0%) and lower than valproate (10.3%). In contrast, in some surveys, there was not any congenital abnormality with phenobarbital. Endo et al [7] conducted a survey including 36 deliveries of 25 mothers with epilepsy. They founded that there was not any congenital abnormality with phenobarbital and the cleft lip with palate was happened in just one case received other agent.
In a recent meta-analysis conducted by Meador et al [8], it has been calculated that phenobarbital is associated with a 4.91% risk of malformation. The risk of other AEDs including Valproate, Phenytoin, Carbamazepine and Lamotrigine were 10.73%, 7.36%, 4.62% and 2.91% respectively.
As well as we know, coloac anomaly has not been reported with phenobarbital use during pregnancy. It is the most complex type of imperforate anus with confluence of the rectum, vagina, and bladder in a urogenital sinus, with a relatively poor functional outcome of the bowel, the genital tract and the urinary tract. Although comprehensive surgical planning may help the children to achieve a reasonable lifestyle [9].
Since the patient received just phenobarbital and folic acid, the most probable cause of this anomaly is phenobarbital. Based on the current recommendations, folic acid is not only safe, but also could prevent the NTDs occurring in general population and in women at high risk due to NTDs in previous pregnancies [10]. In addition to anatomical teratogenesis, phenobarbital exposure was associated with cognitive deficits including reduced IQ [11,12].
It is notable that we can not conclusively link the cloacal anomaly to the phenobarbital, as this anomaly may occur in approximately 1 of 50,000 births. According to the World Health Organization (WHO) classification systems proposed for causality assessment (including certain, probable, possible, unlikely, conditional/unclassified and unassessable/ unclassifiable), this adverse reaction of phenobarbital can be classified as a possible side effect [13].
Base on the current evidences, valporate poses the greatest risk to the fetus among AEDs followed by phenytoin and phenobarbital. The overall risks for carbamazepine and lamotrigine appear to be relatively low [11].
Various mechanisms have been proposed for the teratogenicity of AEDs, including folate-related defects, ischemia, neuronal suppression, free radicals and AED-induces neuronal apoptosis [8]. It has been demonstrated that phenobarbital, like as phenytoin, vigabatrin, and valproate, induces widespread neuronal apoptosis in immature animal brains [14,15]. In addition, interaction if AEDs with susceptible genotypes has been suggested and may explain different susceptibilities of individuals [16].
Another important issue of the presented case is the medication error. As noted, the patient received erratically phenobarbital 300 mg/day instead of the scheduled 100 mg/day dosage. Since the malformation induced by phenobarbital is possibly a dose-dependent phenomenon after 60 mg/day [1,17], using the lowest effective dose is a critical point. It is notable that the Phenobarbital level was not checked during pregnancy. It has been recommended that the blood levels of AEDs including Phenobarbital should be monitored during pregnancy to avoid toxicity, although increase clearance and low levels are a more common complication [18].
Conclusion
In addition to previously recognized risks associated with phenobarbital during pregnancy, cloacal anomaly should be considered as a possible adverse outcome. The case presented might be due to phenobarbital effect or other causes remain further investigation of the problem in a larger and more representative birth population than that described. According to the current evidences, the safer AEDs with the lowest effective dosage (e.g., carbamazepine and lamotrigine) should be used for epileptic patients instead of more toxic agents such as valproate and phenobarbital. Also we recommend closed observation regarding ultrasound and fetal echocardiography for any pregnant woman on anticonvulsant therapy.
2534
References
- Kluger BM, Meador KJ. Teratogenicity of antiepileptic medications. Semin Neurol 2008; 28(3): 328-335.
- Perucca E. Birth defects after prenatal exposure to antiepileptic drugs. Lancet Neurol 2005; 4(11): 781-6.
- Tanaka H, Takada A, Izumi M, et al. Effects of antiepileptic drugs on delivery and early childhood: comparison among monotherapies of valproic acid, phenytoin, carbamazepine and Phenobarbital. Rinsho Shinkeigaku 1991; 31(3): 266-9.
- O’Brien MD, Gilmour-White S. Epilepsy and pregnancy. BMJ. 1993; 21;307(6902):492-5.
- Seip M. Growth retardation, dysmorphic facies and minor malformations following massive exposure to phenobarbitone in utero. Acta Paediatr Scand 1976; 65(5):617-21.
- Holmes LB, Wyszynski DF, Lieberman E. The AED (antiepileptic drug) pregnancy registry: a 6-year experience Arch Neurol 2004; 61(5):673-8.
- Endo S, Hagimoto H, Yamazawa H, et al. Statistics on deliveries of mothers with epilepsy at Yokohama City University Hospital. Epilepsia 2004, 45: 8: 42-70.
- Meador KJ. Cognitive effects of epilepsy and of antiepileptic medications. In: Wyllie E, editor. The Treatment of Epilepsy. Principles and Practices. 4 th edition. Philadelphia: Lippincott Williams & Wilkins. 2005; p 1185-95.
- Hendren WH. Cloaca, the most severe degree of imperforate anus: experience with 195 cases. Ann Surg 1998; 228(3):331-46. 10.
- Pitkin RM. Folate and neural tube defects. Am J Clin Nutr 2007; 85(1):285S-8S.
- Meador Kj, Reynolds MW, Crean S, et al. Pregnancy outcomes in women with epilepsy: A systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res 2008; 18: [Epub ahead of print].
- Bromley RL, Baker GA, Meador KJ. Cognitive abilities and behaviour of children exposed to antiepileptic drugs in utero. Curr Opin Neurol 2009; 22(2):162-66.
- Edwards IR, Biriell C. Harmonisation in pharmacovigilance. Drug Saf 1994; 10(2):93-102.
- Ikonomidou C et al. Ethanol-induced apoptosis neurodegeneration in fetal alcohol syndrome. Science 2000; 287: 1056-60.
- Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci U S A. 2002; 12;99(23):15089-94.
- Finnell RH, Bennett GD, Slattery JT, et al. Effect of treatment with phenobarbital and stiripentol on carbamazepine-induced teratogenicity and reactive metabolite formation. Teratology 1995; 52(6):324-32.
- Jones KL, Johnson KA, Chambers CC. Pregnancy outcome in women treated with phenobarbital monotherapy. Teratology 1992; 45:453-4.
- Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology 2003; 1;61(6 Suppl 2):S35-42.








