Ashwaq Al-Sulami*
Department of Faisal Specialist, Prince Sultan University, Riyadh, Saudi Arabia
- *Corresponding Author:
- Ashwaq Al-Sulami
Department of Faisal Specialist
Prince Sultan University
Riyadh, Saudi Arabia
Tel: 966553641776
E-mail: aalsulami@kfshrc.edu.sa
Received Date: February 05, 2021; Accepted Date: February 19, 2021; Published Date: February 26, 2021
Citation: Sulami AA (2021) Clobazam Efficacy as an Add-on Therapy for 121 Patients with Intractable Epilepsy. J Neurol Neurosci Vol.12 No.3: 357.
Background: Clobazam is a novel 1,5-benzodiazepine that
was initially developed as an antianxiety treatment
designed to decrease adverse Effects associated with 1,4-
benzodiazepines while maintaining efficacy. It was later
found to have antiepileptic properties.
Clobazam is well-tolerated and has shown efficacy as an
adjunctive therapy for a variety of epilepsy types, including
LGS. Studies have reported tolerance rates as high as 87%.
Clobazam has an important antiepileptic effect and is less
expensive than the new antiepileptic drugs, but still has not
been considered as first- line drug in the treatment of
epilepsy. Clobazam have been established as valuable addon
medication for refractory epilepsy. Clobazam have been
used in our institution for more than 15 years; with benefit
observe in many patients.
Methods and Results: Retrospective chart review of patient
who were treated with Clobazam between 2008 and 2017
for treatment of intractable epilepsy. The following
information were collected; age, sex, seizures type and
frequency per month pre-and post Clobazam. Information
was entered in Redcap database. 121 patients were
reviewed to evaluate the response of seizures to the use of
Clobazam. Age mean is 8 years old; male (73) and female
(48). The median decrease in the average number of
monthly seizures for all patients was 30. The seizures with
highest response to Clobazam are myoclonic, atonic,
infantile spasm more than generalized tonic clonic and
partial seizures. The median decrease in the average
monthly seizures following Clobazam was 30 among males
and 40 among females no significant association between
the sex and the decrease of average monthly seizures
following Clobazam. Clobazam presented a stronger effect
on younger children compared to the age above 15.
Conclusions: Our study confirmed the efficacy of Clobazam
for treatment of intractable epilepsy. Clobazam efficacy
more superior in generalized seizures type i.e. myoclonic,
atonic, infantile spasm than GTC and partial seizures.
Younger children response better than older children.
Keywords
Cardiac arrest; Echocardiography; Hypovolemic; Sepsis
Introduction
Clobazam is a novel 1,5-benzodiazepine that was initially developed as an antianxiety treatment designed to decrease adverse Effects associated with 1,4-benzodiazepines while maintaining efficacy [1,2]. It was later found to have antiepileptic properties [2-5]. Clobazam appears to exert its effects by increasing c-amino butyric acid (GABA) mediated inhibitory effects through binding with the a1subunit of the GABA receptor, increasing GABA transporters, and increasing glutamate reuptake [6,7]. Oral absorption of Clobazam is rapid and complete. The time to peak concentration ranges from 1 to 4 hour [8]. The drug is highly lipophilic and is rapidly distributed in fat and cerebral gray matter. Within 14 hours of administration it has accumulated in white matter and is then widely redistributed; the volume of distribution is large [9,10] Clobazam is well-tolerated and has shown efficacy as an adjunctive therapy for a variety of epilepsy types, including LGS [11]. Studies have reported tolerance rates as high as 87%. [12] Clobazam has an important antiepileptic effect and is less expensive than the new antiepileptic drugs, but still has not been considered as first-line drug in the treatment of epilepsy. Clobazam have been established as valuable add-on medication for refractory epilepsy. Clobazam have been used in our institution for more than 15 years; with benefit observe in many patients.
Objective
To Evaluate the Efficacy of Clobazam in Children as Add-on therapy with Refractory Epilepsy in Saudi Arabia.
Methods
Retrospective chart review of patients who were treated with Clobazam as add-on therapy between 2008 and 2017 for intractable epilepsy. In epilepsy clinics of King Faisal Specialist Hospitals and Research center. The following information were collected; age, sex, seizures type and frequency per month pre-and post Clobazam. Clobazam mean dosage was 20 mg/day. 0.9 mg/kg/day (range 0.05-2 mgkgday). Mean use of Clobazam was 20 months. While keeping the previous AEDs regimens constant, the data were entered to Redcap database.
Results
There were a total of 121 epilepsy patients. Age mean is 8 years old; male (73) age ranged 1-17 year and female (48) age ranged 2-22 years old. (Graph 1) with different types of seizures; this figure illustrates the seizure types that were included. (Figure 1) the number of seizures pre-and post Clobazam. The median decrease in the average number of monthly seizures for all patients was 30 seizures per month. This shows a significant decrease with a p-value=0.0001. There were 10 Patients who presented with complex partial seizures. The median decrease in the average monthly number of seizures following Clobazam was 60. This is a statistically significant decrease with a pvalue= 0.0010.
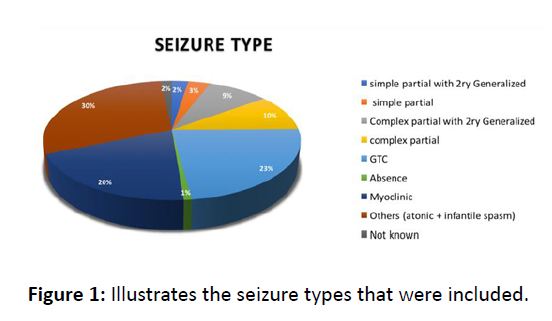
Figure 1: Illustrates the seizure types that were included.
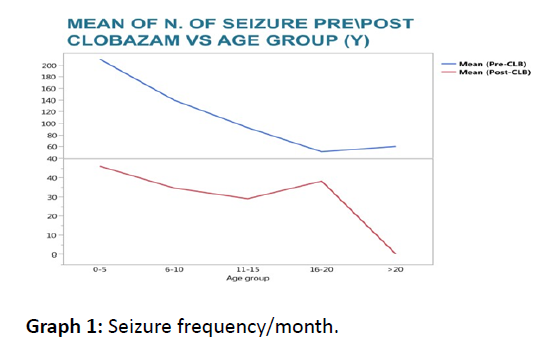
Graph 1: Seizure frequency/month.
Discussion
Kaplan-Meier analysis showed the efficacy was reported in low doses. And the seizure freedom is most likely to be seen in patients on first and second add on therapy with Clobazam. [12] Our study provided the evidence for the same. The percentage of seizure reduction was reported only after the addition of Clobazam while keeping the other treatment regimens constant. Retrospective study on Clobazam efficacy [5] reported that patients with multiple seizure types, nearly 40% of patients had a 50% reduction in the frequency of all their seizure types, including 14% of patients who achieved seizure freedom across all seizure types. In our study revealed the median decrease in the average number of monthly seizures for all patients in all seizure type (myoclonic, atonic, infantile spasm more than generalized tonic clonic and partial seizures) was 30 seizures per month with a p. value 0.0001.Randomized, double-blind study [11] reported imbalance in male-to-female enrollment which was not likely to influence the results of Clobazam efficacy. In comparison to our study there was no effect of gender on Clobazam efficacy in our group. In the literature, no reported relation between specific age group and efficacy to Clobazam. While in our study, CLB was found to be stronger effect on younger children compared to the age above 15 years.
Conclusion
Our study confirmed the efficacy of Clobazam for treatment of intractable epilepsy. Clobazam efficacy more superior in generalized seizures type i.e. myoclonic, atonic, infantile spasm than GTC and partial seizures. Younger children response better than older children.
35969
References
- Chapman AG, Horton RW, Meldrum BS. (1978) Anticonvulsant action of a 1,5- benzodiazepine, clobazam, in reflex epilepsy. Epilepsia 19: 293-299.
- Fielding S, Lal H. (1982) A review of the animal pharmacology of clobazam: An update. Drug Dev Res 2(Suppl 1): 17-21.
- Gastaut H. (1978) Exceptional and unrecognized antiepileptic properties of a commercial anxiolytic: Clobazam. Concours Med 100: 3697-3701.
- Gastaut H, Low MD. (1979) Antiepileptic properties of clobazam, a 1,5- benzodiazepine, in man. Epilepsia 20: 437-446.
- Canadian Clobazam Cooperative Group. (1991) Clobazam in treatment of refractory epilepsy: the Canadian experience. A retrospective study. Epilepsia 32: 407-416.
- Mehndiratta MM, Krishnamurthy M, Rajesh KN, Singh G. (2003) Clobazam monotherapy in drug naive adult patients with epilepsy. Seizure 12: 226-228.
- Doi T, Ueda Y, Tokumaru J, Willmore LJ. (2005) Molecular regulation of glutamate and GABA transporter proteins by clobazam during epileptogenesis in Fe(+++)-induced epileptic rats. Brain Res Mol Brain Res 142: 91-96.
- Rupp W, Badian M, Christ 0. (1979) Pharmacokinetics of single and multiple doses of clobazam in humans. Br J Clin Pharmacol 7: 5S-7S.
- Aucamp AK. (1982) Aspects of the pharmacokinetics and pharmacodynamics of benzodiazepines with particular reference to clobazam. Drug Dev Res 1: 117-126.
- Volz M, Christ 0, Kellner HM.(1979) Kinetics and metabolism of clobazam in animals and man. Br J Clin Pharmacol 7(supp 1): 41S-50S.
- Conry. (2008) Clobazam in the treatment of Lennox-Gastaut syndrome. Epilepsia, 50: 1158-1166.
- Brodie MJ, Barry SJ, Bamagous GA, Norrie JD, Kwan P. (2012) Patterns of treatment response in newly diagnosed epilepsy. Neurology. 78: 1548-1554.







