Keywords
Aloe vera; Anthraquinone; Aloe- emodin; Antimicrobial activity; MIC; Phytochemicals
Introduction
Aloe vera is a shrubby, perennial succulent plant of Liliaceae family having turgid pea-green leaves joined at the stem in a rosette pattern. Aloe vera plant is characterized by stemless large, thick, fleshy leaves having a sharp apex and a spiny margin [1]. Aloe vera has been traditionally used for a variety of medicinal purposes. It is an inseparable part of indigenous medicine system of India. It has gained high importance for its diverse therapeutic properties. Aloe vera contains over 75 nutrients and 200 active compounds; including vitamins, enzymes, minerals, sugars, lignin, anthraquinones, saponins, salicylic acid and amino acids which are responsible for their medicinal properties [2]. Its secondary metabolites have multiple applications such as emollient, purgative, antibacterial, antioxidant, antifungal, antiseptic and in cosmetics industries [3-6]. Aloe vera gel has been used since early times for the topical treatment of various skin conditions such as cuts, burns and eczema [7]. Aloe vera has been shown to have anti inflammatory activity [8,9], immuno stimulatory activity [10] and cell growth stimulatory activity [11]. Furthermore, activity against a variety of infectious agents has been attributed to Aloe vera; for instance antiviral [12] and antifungal [13].
Aloe vera contains free anthraquinones and their derivatives like Barbaloin, Aloe-emodin-9-anthrone, lsobarbaloin, Anthrone-Cglycosides and chromones. In large amounts these compounds exert a powerful purgative effect while in smaller quantity; they appear to aid absorption from the gut therefore it acts as potent antimicrobial agents [14]. Specific plant compounds such as anthraquinones [15,16] and dihydroxy- anthraquinones [17], as well as saponins [18] have been proposed to have direct antimicrobial activity.
Aloe- emodin (1,8-dihydroxy-3-(hydroxymethyl)anthracene- 9,10-dione) derived from leaves of Aloe vera is an antimicrobial compound [19, 20]. It is also known to have anticancerous activity in neuroectodermal tumors [21], lung squamous cell carcinoma [22], hepatoma cells [23], in a glia cell line [24] and a human glioma cell line [25,26]. Present study focuses on antimicrobial activity of methanolic extracts of Aloe vera from 12 different accessions covering 6 agro- climatic regions of India. It also deals with standardization of quantities of antimicrobial compound aloe- emodin from different accession.
Experimental Section
Collection of plant
Samples were collected from 12 sites covering 6 agroclimatic zones of India. Each site had 2 sub-sites. Samples were collected in the months of Jan-Feb 2013. Healthy leaves of Aloe vera were collected from individual plants at each location. Different collection sites have been depicted in Figure 1. Geographical locations and average rainfall of these sites have been given in Table 1. The plant material was identified and authenticated by comparing the herbarium specimen available in Department of Genetics, M. D. University, Rohtak (India). Herbarium specimen number is MDU-6803. Tissues were placed in sterile plastic bags and brought in an ice box, sealed properly. All samples were brought to the laboratory and processed further.
| S.No. |
Accessions names |
Place of collection |
Latitude |
Longitude |
Average rainfall (mm) |
| 1 |
Jammu |
Jammu |
34° 44' N |
78° 54' E |
1,011 |
| 2 |
Himachal Pradesh |
Palampur |
32° 29' N |
78° 10' E |
1,251 |
| 3 |
Punjab |
Sangrur |
30° 40' N |
75° 50' E |
649 |
| 4 |
Haryana |
Rohtak |
30° 30' N |
76° 60' E |
617 |
| 5 |
Rajasthan |
Jaisalmair |
27° 00' N |
74° 00' E |
209.5 |
| 6 |
Gujarat |
Gandhinagar |
23°.00' N |
72°.00' E |
1,107 |
| 7 |
Uttar Pradesh |
Pratapgarh |
27° 40' N |
80° 00' E |
904 |
| 8 |
Madhya Pradesh |
Bhopal |
23° 30' N |
80° 00' E |
1,146 |
| 9 |
West Bengal |
Kolkata |
23° 00' N |
87° 00' E |
1,582 |
| 10 |
Andhra Pradesh |
Hyderabad |
16° 00' N |
80° 00' E |
812.5 |
| 11 |
Goa |
Kochi |
15° 00' N |
74° 25' E |
3,055 |
| 12 |
Kerala |
Vasco |
10° 00' N |
74° 06' E |
3,005 |
Table 1: Geographical locations and average rainfall of plant collection sites
Figure 1: Different collection sites of Aloe vera from different places of India
Preparation of Crude Plant Materials
The collected leaves were chopped into small pieces and shade dried at room temperature. 100 gm dried leaves pieces were grinded and soaked in 60% ethanol solution for 24 hrs. Sulphuric acid and chloroform were added into the extractive and refluxed to remove the chloroform extractive [27]. The extract was prepared by cold percolation method. Stock solution was prepared by dissolving in methanol with the help of rotary shaker. A reddish brown colloid containing aloe extractive was obtained, which is filtered by using Whatman filter paper. After the evaporation of the methanol, a yellowish-brown colloid was obtained as crude extract.
Antimicrobial Evaluation
Reference Strains: Pathogenic reference ATCC (American Type Culture Collection) strains were used for checking activity. 9 bacterial and 2 fungal strains were used for the study. Strains were procured from Post Graduate Institute of Medical Sciences, Rohtak (Haryana). India. Out of 9 bacterial strains, 7 were gram negative viz. Shigella flexneri ATCC 12022, Proteus mirabilis ATCC 43071, Salmonella typhi ATCC 13311, Serratia marcescens ATCC 27137, Klebsiella pneumonia ATCC 700603, Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853 and 2 were gram positive viz. Enterococcus faecalis ATCC 29212 and Staphylococus aureus ATCC 259323. Fungal strains were Candida albicans ATCC 3018 and Aspergillus niger ATCC 282).
In-vitro Antibacterial and Antifungal assay
Antibacterial and antifungal activity was checked by agar well diffusion method. 100 mg of the methanolic extract was dissolved in 1 ml of DMSO and used as test sample. Fresh overnight grown culture was used for inoculums preparation. Inoculums were prepared in peptone water and incubated for 2 hours. Turbidity was adjusted equivalent to 0.5 McFarland units (approximately 108 CFU/ml). Inoculums were spread over fresh nutrient agar plates with a sterile spreader. 4 wells of 6 mm diameter were cut with the help of a sterile cutter. Control disc was placed in centre. 10, 20, 30 and 40 μl of this prepared test sample was added to all bacterial strains used and DMSO was used as negative control. 10 μg streptomycin and 10 μg ketoconazole discs were used as positive control respectively for antibacterial and antifungal activity. Both were purchased from Himedia laboratories Pvt. Ltd (India). Plates were incubated for 24 hours at 37°C. Clear zone of inhibition around each well was measured with help of standard ruler HiAntibiotic ZoneScaleTM-C supplied by HiMedia Laboratories Pvt. Ltd. India. Each experiment was done in triplicates.
Minimum Inhibitory Concentration [MIC]
Minimum inhibitory concentration (MIC) is the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation. The MIC values of extracts were determined based on a micro broth dilution method in 96 multi-well micro titer plates developed by Sarkar et al. [28] with slight modifications. 50 μl of nutrient broth and 50 μl of normal saline were added to each well of plate. 10 μl of resazurin indicator solution (prepared by dissolving a 270 mg tablet in 40 ml of sterile distilled water) was added in each well. A volume of 100 μl of test materials (stock concentration 10 mg/ ml of extracts) was added into the first row of the plate. Serial dilutions were performed such that each well had a total of 100 μl of the test material in serially descending concentrations. Finally 10 μl of bacterial suspension concentration of 5 x 106 CFU/ml was added to each well. Plate had a column with streptomycin as positive control. The plates were prepared in triplicate and placed in an incubator at 37°C for 18 to 24 hours. Any colour change from purple to pink indicates growth of microbes. The highest dilution at which no color change occurred was taken as the MIC value of extract and was expressed in mg/ml.
HPLC Analysis
Chemicals used
Aloe-emodin (used for the control of retention times), HPLC grade methanol, acetonitrile and phosphoric acid were purchased from Sigma-Aldrich (USA). AR grade ethanol, sulfuric acid and chloroform were used for the extract preparation. Ultra pure water (18.2 MΩ·cm−1) was obtained by means of a Milli Q apparatus by Millipore, USA. Stock solutions of the analyte (1 mg/mL) were prepared by dissolving pure substance in methanol and were stable for at least two months when stored at −20°C (as assessed by HPLC assays). Standard solutions were prepared daily by diluting stock solutions with the mobile phase and directly injected into the HPLC system.
Instrumentation
A High Performance Liquid Chromatography equipped with gradient elution capability, Ultraviolet spectrophotometer (UV) and photodiode array (PDA) as detector and an auto sampler (Agilent Technologies system, model 1260 Infinity, USA). A stainless steel C18 column, length of 250 mm, internal diameter 4.6 mm and 5 μm particle size (250 mm × 4.6 mm 5 μm) column was used. The column temperature maintained at ambient condition.
HPLC Analysis Condition
The mobile phase consisted of acetonitrile (A) and 0.1% aqueous phosphoric acid (B) with a gradient elution of 24% A at 0-12 min, 24-50% A at 12-22 min, 50-24% A at 22-40 min and 24% A at 40-50 min. The flow rate was 1.0 ml/min and the separation was monitored by absorbance at 254 nm. The injection volume was 20 μl. The identification of peaks was done by comparing HPLC chromatograms of individuals with the peak of purchased standard aloe- emodin.
Results
Methanolic leaf extracts of Aloe vera were prepared. Yield of extracts have been shown in Table 2. Maximum yield was obtained from Punjab (4.3 gm) and Haryana (4.2 gm) accessions. Minimum yield was from Kerala (3.2 gm). All extracts showed the activity against all reference bacterial and fungal strains. Antimicrobial activities were ranged from 11.50 mm to 20.50 mm as measured by diameter of zone of inhibition. Result of zone of inhibition of all accessions and control against test strains have been given in Table 3. Antibacterial activity for some of the A. vera accessions have been given in Figure 2.
| Agro-climatic zone |
Name of State |
Yield (g) |
| Highland |
Jammu |
4 |
| Himachal Pradesh (HP) |
3.8 |
| Semi-arid |
Haryana |
4.2 |
| Punjab |
4.3 |
| Arid |
Rajasthan |
3.6 |
| Gujarat |
3.5 |
| Humid Subtropical |
Uttar Pradesh (UP) |
4 |
| Madhya Pradesh (MP) |
3.7 |
| Tropical wet & dry |
West Bengal (WB) |
4.1 |
| Andhra Pradesh (AP) |
3.9 |
| Tropical wet |
Goa |
3.4 |
| Kerala |
3.2 |
Table 2:Yield of Aloe vera extracts collected from different Agro- climatic zones
| Accessions |
CA |
AN |
SF |
EF |
SA |
PM |
ST |
SM |
KP |
EC |
PA |
| Jammu |
20.00±10 |
17.83±0.7 |
14.66±0.5 |
14.00±0.5 |
16.83±0.7 |
14.66±0.5 |
16.00±0.5 |
14.16±0.7 |
13.50±0.5 |
15.16±0.7 |
13.00±1.0 |
| HP |
18.16±07 |
18.0±0.5 |
15.83±0.7 |
14.66±0.7 |
16.33±0.5 |
14.83±0.7 |
15.00±0.5 |
12.50±0.5 |
14.00±0.5 |
16.00±1.0 |
12.50±0.5 |
| Punjab |
17.00±05 |
16.16±0.7 |
16.00±1.0 |
14.33±0.5 |
20.00±0.5 |
13.33±0.5 |
18.50±0.5 |
13.00±0.5 |
12.16±0.2 |
19.00±0.5 |
13.83±0.7 |
| Haryana |
19.00±0.5 |
15.66±0.5 |
16.16±0.7 |
13.00±0.5 |
19.00±1.0 |
16.50±0.5 |
19.00±0.5 |
14.16±0.2 |
13.00±0.5 |
20.50±1.0 |
14.50±0.5 |
| Rajasthan |
19.83±0.7 |
17.33±0.5 |
18.00±1.0 |
16.00±1.0 |
17.33±0.5 |
15.5±0.5 |
17.16±0.2 |
13.83±0.7 |
16.16±0.2 |
18.50±0.5 |
14.66±0.5 |
| Gujarat |
17.16±0.7 |
16.50±0.5 |
14.33±0.5 |
14.00±1.0 |
17.00±1.0 |
13.00±0.5 |
18.5±0.5 |
13.50±0.5 |
13.33±0.7 |
19.83±0.7 |
13.66±0.5 |
| UP |
19.50±0.5 |
15.00±1.0 |
14.66±0.5 |
13.5±0.5 |
20.16±0.2 |
14.33±0.5 |
17.33±0.5 |
14.33±0.5 |
13.50±0.5 |
18.33±0.5 |
14.00±1.0 |
| MP |
18.00±1.0 |
11.5±0.5 |
16.00±0.5 |
14.0±0.5 |
18.5±0.5 |
14.33±0.5 |
15.83±0.7 |
12.50±0.5 |
14.50±0.5 |
19.00±1.0 |
12.50±0.5 |
| WB |
17.66±0.5 |
16.0±1.0 |
16.00 ±1.0 |
12.66±0.5 |
19.16±0.7 |
13.00±0.5 |
18.16±0.2 |
13.33±0.7 |
16.00±1.0 |
20.50±0.5 |
13.50±1.0 |
| AP |
19.00±1.0 |
17.50±0.5 |
16.83±0.7 |
13.66±0.5 |
20.83±0.7 |
12.83±0.7 |
17.33±0.7 |
14.00±0.5 |
14.83±0.7 |
19.5±1.0 |
14.00±1.0 |
| Goa |
18.66±0.5 |
16.66±0.5 |
16.00±0.5 |
12.00±1.0 |
20.33±0.5 |
13.00±0.5 |
17.00±0.5 |
13.50±0.5 |
14.33±0.7 |
19.00±1.0 |
11.83±0.7 |
| Kerala |
17.83±0.7 |
15.00±0.5 |
16.16±0.7 |
13.83±0.7 |
20.00±1.0 |
14.83±0.7 |
18.50±0.5 |
14.50±0.5 |
14.00±1.0 |
19.00±0.5 |
13.00±1.0 |
| Control |
18.0±1.0 |
23.0±0.5 |
14.0±1.0 |
23.0±0.5 |
26.0±1.0 |
23.5±0.5 |
24.0±0.5 |
25.5±0.5 |
12.0±1.0 |
13.0±1.0 |
18.0±1.0 |
Fungal strains = CA- Candida albicansand AN- Aspergillusniger
Bacterial strains = EC- Escherichiacoli, PM- Proteus mirabilis, KP- Klebsiellapneumoniae, PA- Pseudomonas aeruginosa, SF- Shigellaflexneri, SM- Serratiamarcescens, ST- Salmonellatyphi, EF- Enterococcusfaecalis and SA- Staphylococusaureus
Control for fungal strains= Ketocanozole (10μg)
Control for bacterial strains= Streptomycin (10μg)
Table 3: Antimicrobial activity of different accessions of Aloe vera Crude extracts measured by zone of inhibition (mm ± SD)
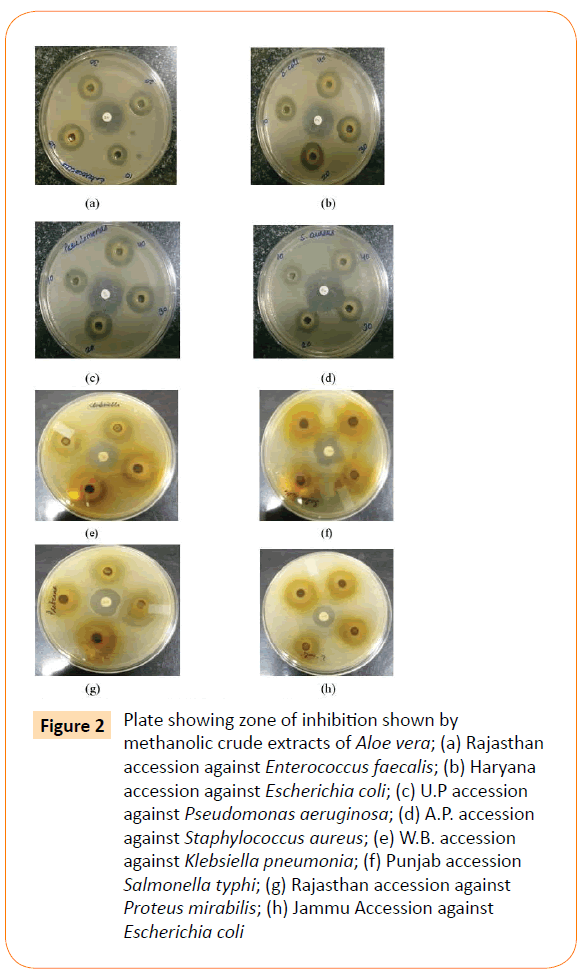
Figure 2: Plate showing zone of inhibition shown by methanolic crude extracts of Aloe vera; (a) Rajasthan accession against Enterococcus faecalis; (b) Haryana accession against Escherichia coli; (c) U.P accession against Pseudomonas aeruginosa; (d) A.P. accession against Staphylococcus aureus; (e) W.B. accession against Klebsiella pneumonia; (f) Punjab accession Salmonella typhi; (g) Rajasthan accession against Proteus mirabilis; (h) Jammu Accession against Escherichia coli
Maximum antibacterial activity was noted against E. coli with a zone of inhibition of 20.50 mm and minimum activity was against P. aeruginosa with 11.83 mm zone of inhibition (Table 3, Figure 2). Activity against Shigella flexneri Klebsiella pneumoniae and E. coli was quiet good as compared to control. Extracts were more active against fungus C. albicans than A. niger. Punjab, Haryana and West Bengal accessions showed good activity against most of the tested strains. Significant activity was reported against K. pneumonia with a maximum 16 mm zone of inhibition by West Bengal accession. Control showed only 12 mm zone of inhibition against this bacteria. Minimum activity was shown against P. aeruginosa, S. marcescens, S. typhi, P. mirabilis and E. faecalis as compared with control. Jammu and HP accessions showed good antifungal activity. Extracts were more active against gram negative bacteria than gram positive ones. MIC values ranged from 1.2 mg/ml to 5 mg/ml. Minimum MIC value of 1.2 mg/ml was found against E. coli revealed by most of the accessions. Maximum MIC was against Proteus mirabilis, Pseudomonas aeruginosa and fungus A. niger shown by most of the accessions. MIC values of all the accessions have been shown in Table 4.
| Accessions |
CA |
AN |
SF |
EF |
SA |
PM |
ST |
SM |
KP |
EC |
PA |
| Jammu |
1.2 |
2.5 |
2.5 |
2.5 |
1.2 |
2.5 |
1.2 |
1.2 |
2.5 |
1.2 |
2.5 |
| HP |
1.2 |
2.5 |
2.5 |
2.5 |
1.2 |
2.5 |
1.2 |
1.2 |
2.5 |
1.2 |
2.5 |
| Panjab |
2.5 |
5.0 |
2.5 |
2.5 |
1.2 |
5.0 |
2.5 |
2.5 |
2.5 |
2.5 |
5.0 |
| Haryana |
2.5 |
5.0 |
2.5 |
2.5 |
1.2 |
5.0 |
2.5 |
1.2 |
2.5 |
1.2 |
5.0 |
| Rajasthan |
2.5 |
5.0 |
2.5 |
2.5 |
1.2 |
5.0 |
1.2 |
1.2 |
2.5 |
1.2 |
5.0 |
| Gujarat |
5.0 |
5.0 |
5.0 |
2.5 |
5.0 |
5.0 |
2.5 |
5.0 |
2.5 |
2.5 |
5.0 |
| UP |
1.2 |
5.0 |
5.0 |
2.5 |
1.2 |
5.0 |
2.5 |
1.2 |
2.5 |
1.2 |
5.0 |
| MP |
2.5 |
5.0 |
2.5 |
2.5 |
2.5 |
5.0 |
2.5 |
5.0 |
2.5 |
1.2 |
5.0 |
| WB |
5.0 |
5.0 |
2.5 |
2.5 |
1.2 |
5.0 |
1.2 |
2.5 |
2.5 |
1.2 |
5.0 |
| AP |
2.5 |
5.0 |
5.0 |
2.5 |
5.0 |
5.0. |
2.5 |
5.0 |
2.5 |
2.5 |
5.0 |
| Goa |
5.0 |
5.0 |
5.0 |
2.5 |
1.2 |
5.0 |
1.2 |
5.0 |
2.5 |
1.2 |
5.0 |
| Kerala |
5.0 |
5.0 |
5.0 |
2.5 |
1.2 |
5.0 |
1.2 |
2.5 |
2.5 |
1.2 |
2.5 |
Fungal strains = CA- Candida albicansand AN- Aspergillusniger
Bacterial strains = EC- Escherichiacoli, PM- Proteus mirabilis, KP- Klebsiellapneumoniae,
PA- Pseudomonas aeruginosa, SF- Shigellaflexneri, SM- Serratiamarcescens, ST- Salmonellatyphi,
EF- Enterococcusfaecalis and SA- Staphylococusaureus
Table 4: Minimum Inhibitory Concentration (MIC in mg/ml) of various accessions of Aloe vera Extracts against different micro- organisms
The HPLC analysis revealed that there was mark able difference in the quantity of aloe- emodin from different accessions that were collected throughout India on the basis of their different climatic zones. The Results obtained indicated the presence of anthraquinone derivative aloe- emodin in each accession with the significant differences in their quantities. Important aspects like Sample name, Climatic zone, Retention time (RT), Area of peak and Yield percentages of compound (aloe- emodin) of HPLC analysis are given in the Table 5. HPLC chromatograms of standard along with 3 accessions viz Gujarat, Uttar Pradesh and Haryana have been shown in Figures 3a-3d.
| Agro-climatic zones |
Sample Name |
RT (min.) |
Area (Mau*s) |
Yield (%) |
| Highland |
Jammu |
28.21 |
112.23 |
0.0826 |
| Himachal Pradesh (HP) |
28.06 |
107.82 |
0.0793 |
| Semi- Arid |
Punjab |
28.21 |
220.25 |
0.1621 |
| Haryana |
28.91 |
129.11 |
0.095 |
| Arid |
Rajasthan |
28.13 |
27.84 |
0.0205 |
| Gujarat |
28.17 |
25.24 |
0.0186 |
| Humid- Subtropical |
Uttar Pradesh (UP) |
28.13 |
154.15 |
0.1134 |
| Madhya Pradesh (MP) |
28.17 |
80.35 |
0.0591 |
| Tropical wet and dry |
West Bengal (WB) |
28.18 |
91.15 |
0.0671 |
| Andhra Pradesh (AP) |
28.2 |
45.83 |
0.0337 |
| Tropical wet |
Goa |
28.22 |
204.53 |
0.1505 |
| Kerala |
28.25 |
99.88 |
0.0735 |
| Standard |
Aloe- emodin |
28.1 |
135779 |
99 |
Table 5: HPLC analysis of different Aloe vera accessions
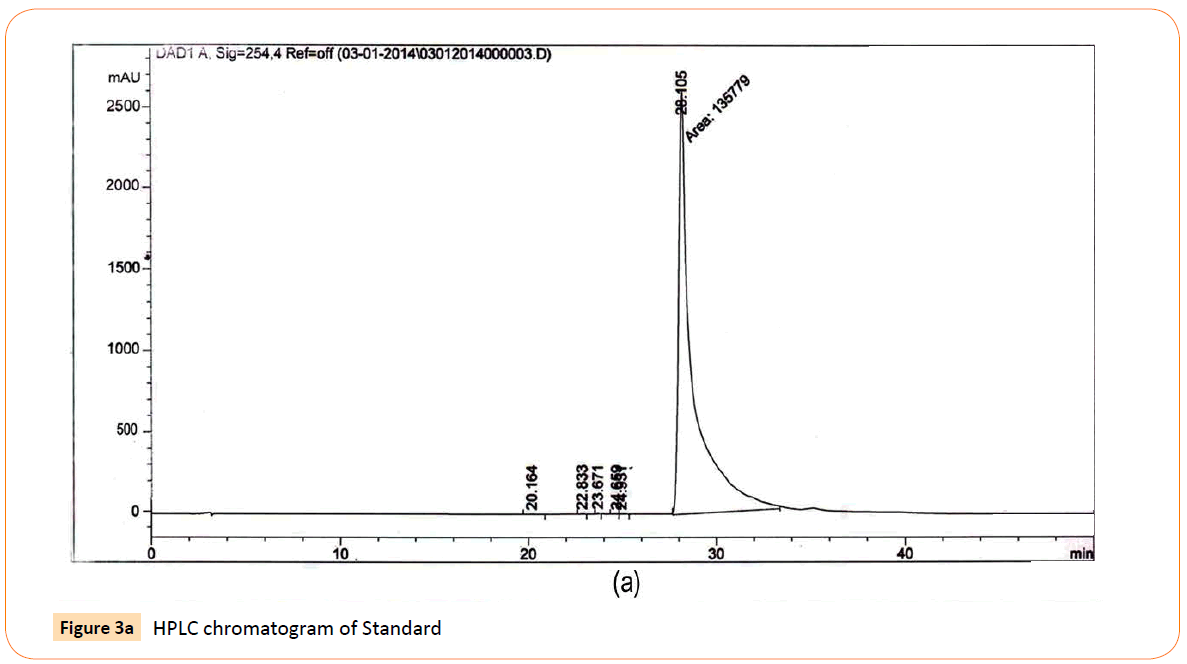
Figure 3a: HPLC chromatogram of Standard
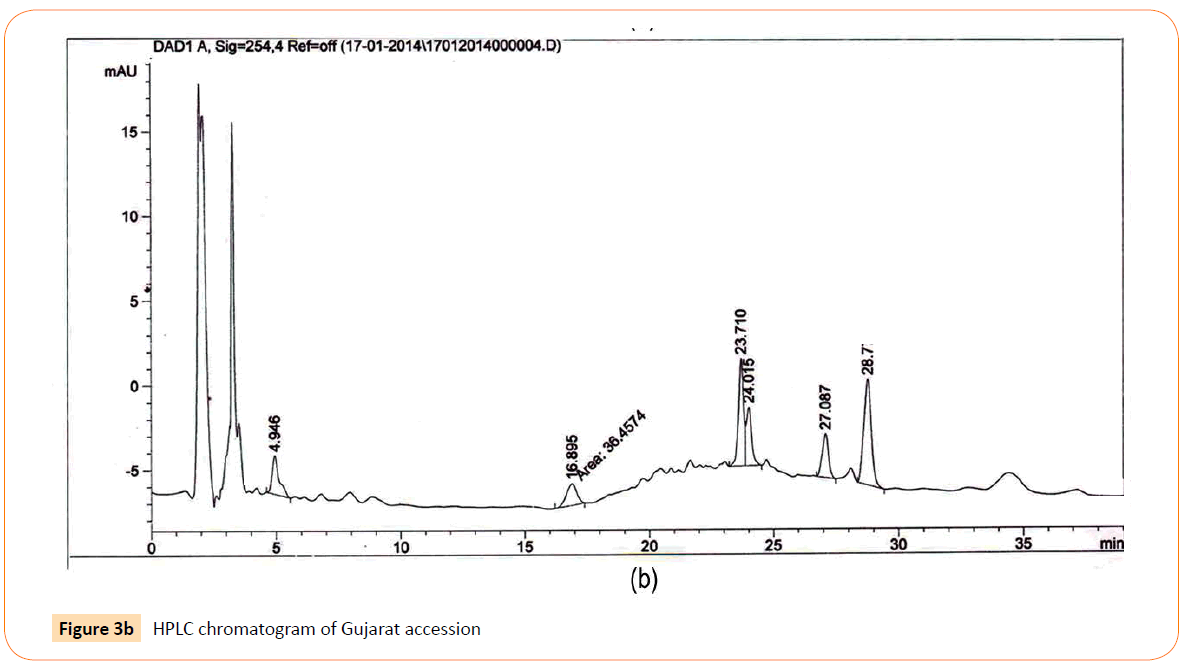
Figure 3b: HPLC chromatogram of Gujarat accession
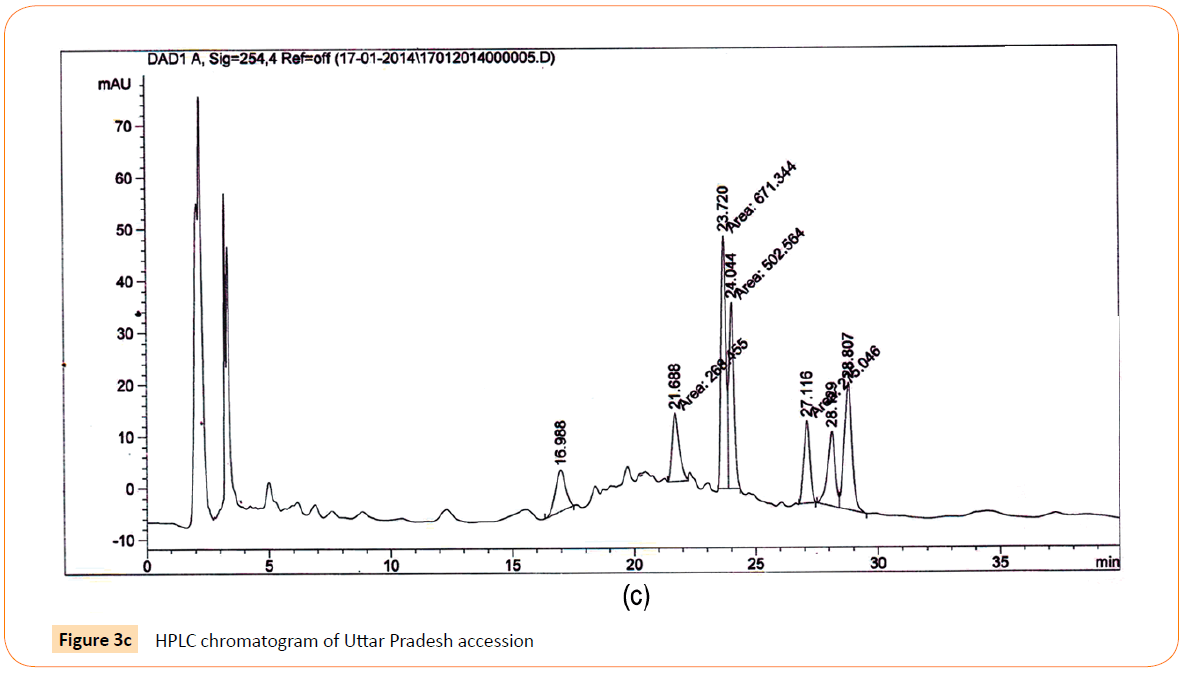
Figure 3c: HPLC chromatogram of Uttar Pradesh accession
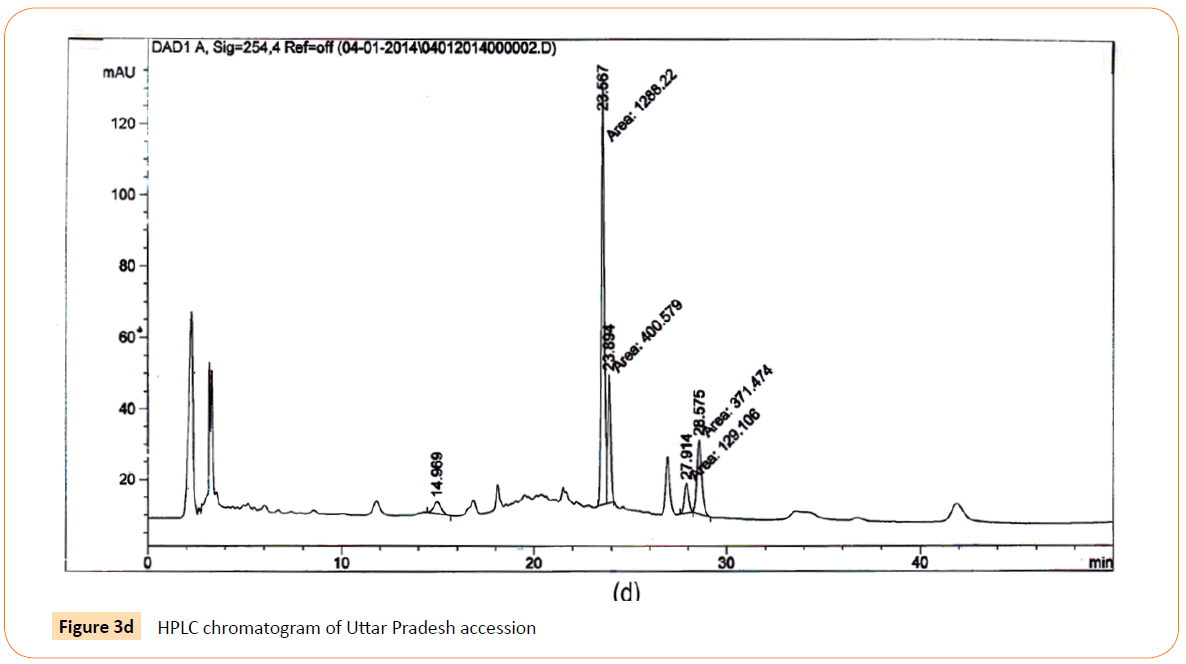
Figure 3d: HPLC chromatogram of Uttar Pradesh accession
RT’s (retention time) of standard was 28.10 min. and all the desired peaks ranged from 28.06 to 28.91 min. Area of desired peaks ranged from 25.24 to 220.25 Mau*s (milli- absorbance unit). Maximum compound percentage was from the Punjab (Semi- arid zone) accession (0.16%) and minimum from the AP (Tropical wet and dry zone) accession (0.03%) as compared with standard (99%). The percentage of aloe-emodin in different accessions has been shown in Figure 4.
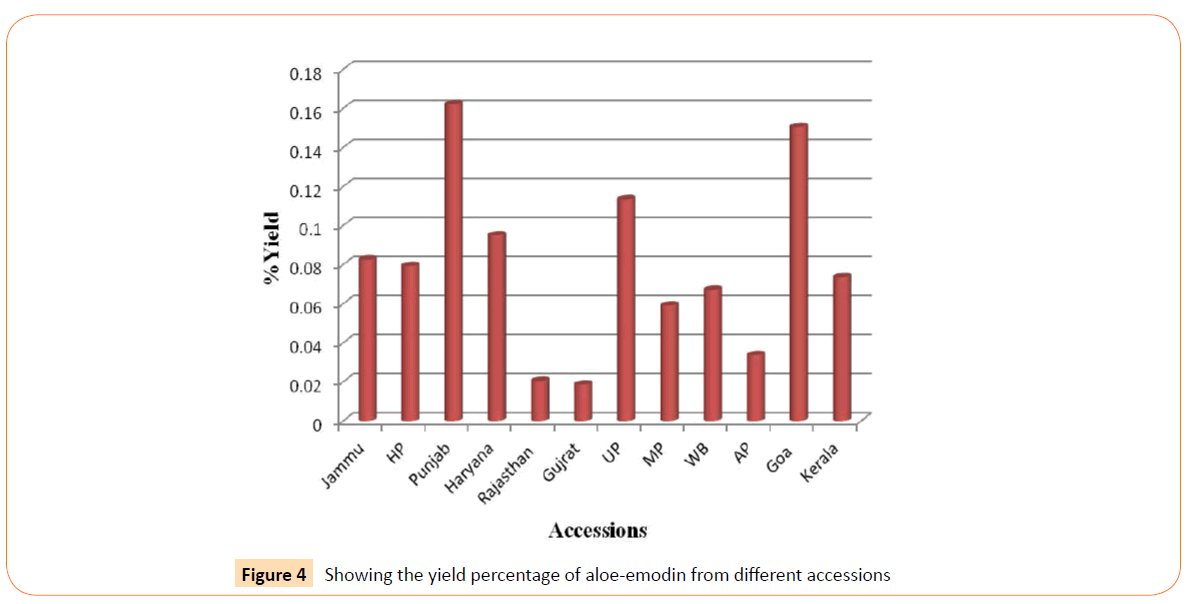
Figure 4: Showing the yield percentage of aloe-emodin from different accessions
Discussion
The process of investigating plants to identify the chemical substances is of great interest to plant scientists because there is a need to discover new drugs for treating diseases. Secondary metabolites formed by living systems, notably from plant origin, have shown great potential in treating human diseases such as cancer, coronary heart diseases, diabetes and infectious diseases [29]. These usually consist of the phenolic and polyphenolic compounds, alkaloids, tannins, saponins, carbohydrates, glycosides, flavonoids and steroids distributed throughout the plant kingdom [30,31].
Aloe vera can grow in almost all types of environmental conditions but there are several factors that can influence the quality and quantity of a particular constituent. The type of solvents and methods of preparation affect antimicrobial activity of plants [32]. In present study, we prepared A. vera leaf gel extracts by using methanol as a solvent. Previous studies report that methanol as a solvent extracts revealed the antimicrobial properties of plants due to presence of phenolic compounds, saponin, bryophyllin and other secondary metabolites which are reported to be antimicrobial [33,34]. Methanolic extract induced the best extraction yield and more complex composition of phenolics [35].
Aloe vera plant is rich in alkaloids, tannins, saponins, flavonids, anthraquinones, barbaloins, glycosides and terpenoids [36]. So methanol is the appropriate solvent for preparation of extracts for antimicrobial study. Our Results correlate with previous studies. Methanol extracts of all 12 accessions exhibited quite good antimicrobial activity. Previous studies on Aloe vera leaf gel extracts showed that extract was more effective against gram positive bacteria than gram negative [37,38]. In our study methanolic extract showed good activity against gram positive S. aureus and gram negative E. coli and S. typhi. Our extracts also showed good activity against both the fungal strains i.e. A. niger and C. albicans.
Antimicobial activity
Although all extracts were quite effective against all the tested strains. But there are variations in diameter of zones of inhibition. Solvent system and extraction procedure was uniform throughout the study. Variation in inhibition zones may be the due to differences in the phytochemical composition of different accessions. Accessions were from 6 climatic zones of India.
Variations in the sensitivity of the bacterial and fungal species tested on the extracts might be because of differences in the local environmental factors. Previous studies states that mineral and phytochemical composition of plants is influenced by various environmental factors including the geography, climate, soil minerals, grazing stress, seasonal changes, phenological stages and ability of plant uptake of minerals from soil [39-41].
A good antimicrobial activity was also shown by Jammu and H.P. accessions. Aloe vera is a cold sensitive plant. During stress more phytochemicals are produced in plants to withstand the adverse conditions. Studies conducted on plants in stress conditions showed higher production of flavonoids, anthocyanins and mucilaginous substances in stress condition [42]. Flavonoids and anthocyanins are both antimicrobial in nature [43].
Quantification of Aloe- emodin
Standardization and characterization of herbal drugs is a topic of continuous scientific interest in the herbal drug industry. With the advent of modern chromatographic systems there is an ever increasing intent to produce and develop easy, rapid, convenient and cost effective methods for standardization [44]. Standardization of methanolic extract of plant leaves requires HPLC which is a sensitive and accurate tool widely used for the quality assessment of plant extract and its derived products and formulation [45]. The High Performance Liquid Chromatography represents the best technique used now days to separate, to fingerprint qualitatively and to identify or quantify each molecule from complex mixtures as is it the case of plant extracts and herbal supplements [46]. The use of multiple methods involving different mobile gradient phases would increase the validity and reliability of the obtained Results.
There are not too much previous comparative studies on diversity bases which can correlates the marker compound analysis of Aloe vera. The Results indicated that, Aloe vera plant collected from humid- subtropical and semi- arid zone showed better aloe- emodin production as compared to tropical area zone. Cassia, another plant genus which is a important source of naturally occurring bioactive compounds anthraquinones also found in tropical and sub tropical areas throughout the world [47]. It supports the statement that subtropical environment conditions favor the anthraquinones production from the plants. It is known that genetic and environmental factors and their interactions affect the pharmaceutically important secondary metabolites in medicinal plants [48]. A variety of environmental factors; such as altitude, radiation, and soil nutrition; have been proven to significantly influence the secondary metabolite profile [49,50]. Furthermore, soil type, sun exposure, temperature and rain fall also have major effects on the phenol content of plants. The variations in the anthraquinone glycoside content could be affected due to the different altitude where the plant was grown [51].
Modern world is moving back towards herbal drugs. But standardization of herbal drugs with respect to concentration of its active principles is a must for their approval in modern medicine system. We have standardized an antimicrobial flavanol aloe- emodin from methanolic extracts of Aloe vera. Present study emphasizes that amount of aloe- emodin in extracts which may be related to the antimicrobial activity shown. Punjab, Haryana, UP and West Bengal showed significant correlation between percentage of presence of marker compound and antimicrobial activity. More marker antimicrobial compounds should be screened for formulation of an herbal drug with wider acceptability from Aloe vera. It will help in commercialization of plant in particular areas with desired pharmacological effects.
Conclusion
Aloe vera is a plant of great interest for the research purposes but most of works have been done on pharmaceutical level. Phytochemical composition of plants is influenced by various environmental and climatic factors. The present study has also showed the role of climatic conditions at different geographical locations on the presence of bioactive component aloe- emodin in crude extracts of Aloe vera due to which it may have good antibacterial activity. It is hoped that this study would lead to the establishment of some compounds that could be used to formulate new and more potent antimicrobial drugs of natural origin.
Conflict of Interest
There is no conflict of interest between authors.
Acknowledgement
Financial Assistance from UGC, New Delhi in the form of UGC-SAP grant (No. F.3-20/2012, SAP II) and UGC-BSR fellowship is gratefully acknowledged.
6496
References
- Steenkamp, V., Stewart, MJ. Medicinal applications and toxicological activities of Aloe products. Pharm Biol 2007; 45: 411-420.
- Park, YI., Jo, TH. Perspective of industrial application of Aloe vera. In: Park YI and Lee SK editors. New Perspective on Aloe. Springer Verlag: New York, USA. 2006; 191-200.
- Khanam, N., Sharma, GK. A critical review on antioxidant and antimicrobial properties of Aloe vera L. Int J Pharm Sci Res 2013; 4: 3304-3316.
- Kumar, S., Yadav, JP. Ethnobotanical and pharmacological properties of Aloe vera: A review. J Med Plant Res 2014; 8: 1387-1398.
- Ramasubramanian, TS., Sivakumar, VT., Thirumalai, AV. Antimicrobial activity of Aloe vera (L.) Burm. f. against pathogenic microorganisms. J Bio Sci Res. 2010; 4: 251–8.
- Arunkumar, S., Muthuselvam, M. Analysis of phytochemical constituents and antimicrobial activities of Aloe vera L. against clinical pathogens. World J Agric Sci. 2009; 5: 572-576.
- Heggie, S., Bryant, GP., Tripcony, L., Keller, J., Rose, P., et al. A Phase III study on the efficacy of topical Aloe vera gel on irradiated breast tissue. Cancer Nurs 2002; 25: 442-451.
- Afzal, M., Ali, M., Hassan, RA., Sweedan, N., Dhami, MS. Identification of Some Prostanoids in Aloe vera Extracts. Planta Med 1991; 57: 38-40.
- Malterud, KE., Farbrot, TL., Huse, AE., Sund, RB. Antioxidant and radical scavenging effects of anthraquinones and anthrones. Pharmacology 1993; 47: 77-85.
- Ramamoorthy, L., Tizard, IR. Induction of apoptosis in a macrophage cell line RAW 264.7 by acemannan, a beta-(1,4)-acetylated mannan. Mol Pharmacol 1998; 53: 415-421.
- Tizard, ID., Busbee, B., Maxwell., Mc, K. Effect of acemannan, a complex carbohydrate, on wound healing in young and aged rats. Wounds 1994; 6: 201-209.
- Kahlon, JB., Kemp, MC., Yawei, N., Carpenter, RH., Shannon, WM., et al. In-vitro evaluation of the synergistic antiviral effects of acemannan in combination with azidothymidine and acyclovir. Mol Biother 1991; 3: 214-223.
- Kawai, K., Beppu, H., Simpo, K., Chihara, T., Yamamoto, N., et al. In-vivo effects of Aloe arborescens Miller var natalensis Berger (Kidachi aloe) on experimental Tinea Pidis in guinea pig feet. Phytother Res 1998; 12: 178-182.
- Joseph, B., Raj, SJ. Pharmacognostic and phytochemical properties of Aloe vera Linn -An overview. Int J Pharma Sci Rev Res 2010; 4: 106-110.
- Garcia-Sosa, K., Villarreal-Alvarez, N., Lubben, P., Pena-Rodriguez, LM. Chrysophanol, an antimicrobial anthraquinone from the root extract of Colubrina gregii. J. Mex. Chem. Soc 2006; 50: 76-78.
- Dabai, YU., Muhammad, S., Aliya, BS. Antibacterial activity of anthraquinone fraction of Vilten doniana. Pakistan J. Biol. Sci 2007; 10: 1-3.
- Wu, YW., Ouyang, J., Xiao, XH., Gao, YW., Liu, Y. Antimicrobial properties and toxicity of anthraquinones by micro calorimetric bioassay. Chinese. J. Chem 2006; 24: 45-50.
- Reynolds, T., Dweck, AC. Aloe vera leaf gel: a review update. J Ethnopharmacol 1999; 68: 3-37.
- Dutta, A., Bandyopadhyay, S., Mandal, C., Chatterjee, M. Aloe vera leaf exudate induces a caspase-independent cell death in Leishmania donovani promastigotes. J Med Microbiol 2007; 56: 629-636.
- Eshun, K., He, Q. Aloe vera: a valuable ingredient for the food, pharmaceutical and cosmetic industries--a review. Crit Rev Food Sci Nutr 2004; 44: 91-96.
- Pecere, T., Gazzola, MV., Mucignat, C., Parolin, C., Vecchia, FD., et al. Aloe-emodin is a new type of anticancer agent with selective activity against neuroectodermal tumors. Cancer Res 2000; 60: 2800-2804.
- Lee, HZ. Protein kinase C involvement in aloe-emodin- and emodin-induced apoptosis in lung carcinoma cell. Br J Pharmacol 2001; 134: 1093-1103.
- Kuo, PL., Lin, TC., Lin, CC. The antiproliferative activity of aloe-emodin is through p53-dependent and p21-dependent apoptotic pathway in human hepatoma cell lines. Life Sci 2002; 71: 1879-1892.
- Yi, J., Yang, J., He, R., Gao, F., Sang, H., et al. Emodin enhances arsenic trioxide-induced apoptosis via generation of reactive oxygen species and inhibition of survival signaling. Cancer Res 2004; 64: 108-116.
- Acevedo-Duncan, M., Russell, C., Patel, S., Patel, R. Aloe-emodin modulates PKC isozymes, inhibits proliferation, and induces apoptosis in U-373MG glioma cells. Int Immunopharmacol 2004; 4: 1775-1784.
- Lin, HJ., Chao, PD., Huang, SY., Wan, L., Wu, CJ., et al. Aloe-emodin suppressed NMDA-induced apoptosis of retinal ganglion cells through regulation of ERK phosphorylation. Phytother Res 2007; 21: 1007-1014.
- Tan, ZJ, Li, FF, Xu, XL. Isolation and Purification of Aloe Anthraquinones Based on an Ionic Liq-uid/Salt Aqueous Two-Phase System. Separ Sci Technol 2011; 98: 150-157.
- Sarker, SD., Nahar, L., Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007; 42: 321-324.
- Lai, HY., Lim, YY., Kim, KH. Blechnum orientale Linn - a fern with potential as antioxidant, anticancer and antibacterial agent. BMC Complement Altern Med 2010; 10: 15.
- Naik, GH., Priyadarsini, KI., Hari, M. Free radical scavenging reactions and phytochemical analysis of Triphala, an ayurvedic formulation. Curr Sci 2006; 90: 1100-1105.
- Sati, SC., Sati, N., Rawat, U., Sati, OP. Medicinal plants as a source of antioxidants. Res J Phytochem 2010; 4: 213-224.
- Eloff, JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol 1998; 60: 1-8.
- Cowan, MM. Plant products as antimicrobial agents. Clin Microbiol Rev 1999; 12: 564-582.
- Okwu, DE., Josiah, C. Evaluation of the chemical composition of two Nigerian medicinal plants. Afr J Biotech 2006; 5: 257-361.
- Pop, RM., Csernatoni, F., Ranga, F., Fetea, F., Socaciu, C. HPLC-UV analysis coupled with chemometry to identify phenolic biomarkers from medicinal plants, used as ingredients in two food supplement formulas. B UASVM Food Science Technol 2013; 70: 99-107.
- Moses, A., Bernard, S., Oriko, OR., Edward A. Preliminary Qualitative Analysis of Phytochemical Constituents of the Endemic Aloe tororoana Raynolds in Tororo, Eastern Uganda. Glo Adv Res J Agric 2014; Sci 3: 096-099.
- Manimaran, S., Loganathan, V., Akilandeswari, S., Jaswanth, a., Sathya, S., et al. Wound healing andantimicrobial activity of formulated cream of leaf volatile oil of Atalantiamonophylla correa. Hamdard 1998; 15: 59-62.
- Ayoola, GA., Johnson, OO., Adelowotan, T., Aibinu, IE., Adenipekun, E., et al. Evaluation of the chemicalconstituents and antimicrobial activity of the volatile oil of Citrus reticulata fruit(Tangerine fruit peel) from South West Nigeria. Afr J Biotech 2008; 7: 2227-2231.
- Ganskopp, D., Bohnert, D. Mineral concentration dynamics among 7 northern Great Basin grasses. J Range Manage 2003; 56: 174-184.
- Khan, ZI., Ashraf, M., Valeem, EE. Forage mineral status evaluation: the influence of pastures. Pak. J. Bot 2006; 38: 1043-1054.
- Hussain, J., Khan, AL., Rehman, N., Zainullah Hussain, ST., Khan, F., et al. Proximate and nutrient analysis of selected medicinal plant species of Pakistan. Pak. J. Nut 2009; 8: 620-624.
- Kaplan F, Kopka J, Sung DY, Zhao W, Popp M, Porat R, Guy CL (2007) Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. The Plant Journal 50: 967-981.
- Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H (2011) Phytochemical screening and Extraction: A Review. IPS 1: 98-106.
- Jain, M., Kapadia, R., Albert, S., Mishra, SH. Standardization of Feronia limonia L. leaves by HPLC, HPTLC, physicochemical and histological parameters. Boletín Latin oamericano y del Caribe de Plantas Medicinales y Aromáticas 2011; 10: 525-535.
- Giri, L., Andola, HC., Purohit, VK., Rawat, MSM., Rawal, RS., et al. Chromatographic and spectral fingerprinting standardization of traditional medicines: an overview as modern tools. Res J Phytochem 2010; 4: 234-241.
- Mattoli, L., Cangi, F., Ghiara, C., Burico, M., Maidecchi, A., et al. A metabolite fingerprinting for the characterization of commercial botanical dietary supplements. Metabolomics 2011; 7: 437-445.
- Dave, H., Ledwani, L. A review on anthraquinones isolated from cassia species and their applications. Indian J Nat Prod Resour 2012; 3: 291-319.
- Hartmann, T. From waste products to ecochemicals: fifty years research of plant secondary metabolism. Phytochemistry 2007; 68: 2831-2846.
- Ramakrishna. A., Ravishankar, GA. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal Behav 2011; 6: 1720-1731.
- Selvamani, P., Sen, DJ., Gupta, JK. Pharmacognostical standardization of Commiphora berryi (Arn) Engl and phytochemical studies on its crude extracts. African J Pharma Pharmacol 2009; 3: 37-46.
- Wang, Z., Ma, P., Xu, L., He, C., Peng, Y., et al. Evaluation of the content variation of anthraquinone glycosides in rhubarb by UPLC-PDA. Chem Cent J 2013; 7: 170.












