Key words
|
| |
| Clitoria ternatea, Anthelmintic, Eisenia foetida, Levamisole |
| |
INTRODUCTION
|
| |
| Helminth derived from the Greek word “helminths” meaning “worm”. Helminth is a broad categorical term referring to various types of parasitic worms that reside in the body [1]. The World Health Organization reveals that over two billion people are suffering from parasitic worm infections [2]. It is estimated that by the year 2025, about 57% of the population in developing countries will be influenced [3]. The prevalence of parasitic helminths typically displays a negative binomial distribution within an infected population such that relatively few persons carry heavy parasite burdens. Without treatment, those individuals are most likely to become ill and to perpetuate infection within their community [4]. Anthelmintics are drugs that may act locally to expel worms from the GIT or systemically to eradicate adult helminths or development forms that invade organs and tissues [5]. Most of the existing anthelmintics produce side effects such as abdominal pain, loss of appetite, nausea, Vomiting, headache and diarrhoea [6]. Anthelmintics from the natural sources may play a key role in the treatment of these parasite infections [7]. Increasing problems of development of resistance in helminths against anthelmintics have led to the proposal of screening medicinal plants for their anthelmintic activity [8]. Clitoria ternatea is commonly known as Aparajita and belong to family Fabaceae is cultivated throughout India. The plant is reported to possess to including antimicrobial and insecticidal [9], nootropic, anxiolytic, antidepressant, antistress and anticonvulsant activities [10], hepatoprotective activity, antidiabetic, sedative and blood platelet aggregation-inhibiting properties. In Ayurveda, the roots, seeds and leaves of Clitoria ternatea have long been widely used as a brain tonic and is believed to promote memory and intelligence. Clitoria ternatea has been traditionally used as an anthelmintic [11]. |
| |
| However, anthelmintic activity of Clitoria ternatea leaves has not so far been scientifically proved, so the present study was carried out to assess the anthelmintic activity of Clitoria ternatea against Eisenia foetida by using its two extracts aqueous and ethanolic extract. |
| |
MATERIALS AND METHODS
|
| |
|
Plant collection and authentication
|
| |
| The leaves of Clitoria ternatea was collected from Ajmer, Rajasthan, India and authenticated by Dr. Saroj Arora, Guru Nanak Dev University Amritsar and the specimen voucher No. is 0406/HRB. |
| |
|
Worms Collection and authentication
|
| |
| Eisenia foetida was collected from the water logged areas of the soil and identified and authenticated at the Ujjwal Ujala Vermiculture Group, Vill. Ibban Kalan, Amritsar and its Ref. No. is UU/1210/AL-34. |
| |
|
Preparation of Extract Aqueous extract preparation:
|
| |
| The crude aqueous extract of the Clitoria ternatea leaves was prepared according to the standard method. One hundred grams of the powdered plant material was mixed with 500 mL of distilled water in a Soxhlet apparatus for 8-12 h. The filtrate was then concentrated in a rotary evaporator and the extract stored at 4ºC until required. |
| |
|
Ethanolic extract preparation:
|
| |
| The powder leaves material of Clitoria ternatea was placed in a thimble and extracted with 70% ethanol in a Soxhlet apparatus for 8-12 h. Solvents were removed at temperature below 50ºC in an oven. The residue (extract) of respective plant material was stored at 4°C until used. |
| |
| Phytochemical evaluation: Phytochemical examinations were carried out for all the extracts as per the standard methods. |
| |
| 1. Detection of alkaloids: Extracts were dissolved individually in dilute Hydrochloric acid and filtered. |
| |
| a) Mayer’s Test: Filtrates were treated with Mayer’s reagent (Potassium Mercuric Iodide). Formation of a yellow coloured precipitate indicates the presence of alkaloids. |
| |
| b) Wagner’s Test: Filtrates were treated with Wagner’s reagent (Iodine in Potassium Iodide). Formation of brown/reddish precipitate indicates the presence of alkaloids. |
| |
| c) Dragendroff’s Test: Filtrates were treated with Dragendroff’s reagent (solution of Potassium Bismuth Iodide). Formation of red precipitate indicates the presence of alkaloids. |
| |
| d) Hager’s Test: Filtrates were treated with Hager’s reagent (saturated picric acid solution). Presence of alkaloids confirmed by the formation of yellow coloured precipitate. |
| |
| 2. Detection of carbohydrates: Extracts were dissolved individually in 5 ml distilled water and filtered. The filtrates were used to test for the presence of carbohydrates. |
| |
| a) Molisch’s Test: Filtrates were treated with 2 drops of alcoholic á-naphthol solution in a test tube and conc. Sulphuric acid was added Formation of the violet ring at the junction indicates the presence of Carbohydrates. |
| |
| b) Benedict’s Test: Filtrates were treated with Benedict’s reagent and heated gently. Orange red precipitate indicates the presence of reducing sugars. |
| |
| c) Fehling’s Test: Filtrates were hydrolysed with dil. HCl, neutralized with alkali and heated with Fehling’s A & B solutions. Formation of red precipitate indicates the presence of reducing sugars. |
| |
| 3. Detection of glycosides: Extracts were hydrolysed with dil. HCl, and then subjected to test for glycosides. |
| |
| a) Modified Borntrager’s Test: Extracts were treated with Ferric Chloride solution and immersed in boiling water for about 5 minutes. The mixture was cooled and extracted with equal volumes of benzene. The benzene layer was separated and treated with ammonia solution. Formation of rosepink colour in the ammonical layer indicates the presence of anthranol glycosides. |
| |
| b) Legal’s Test: Extracts were treated with sodium nitropruside in pyridine and sodium hydroxide. Formation of pink to blood red colour indicates the presence of cardiac glycosides. |
| |
|
4. Detection of saponins
|
| |
| a) Froth Test: Extracts were diluted with distilled water to 20ml and this was shaken in a graduated cylinder for 15 minutes. Formation of 1 cm layer of foam indicates the presence of saponins. |
| |
| b) Foam Test: 0.5 gm of extract was shaken with 2 ml of water. If foam produced persists for ten minutes it indicates the presence of saponins. |
| |
|
5. Detection of phytosterols
|
| |
| a) Salkowski’s Test: Extracts were treated with chloroform and filtered. The filtrates were treated with few drops of Conc. Sulphuric acid, shaken and allowed to stand. Appearance of golden yellow colour indicates the presence of triterpenes. |
| |
| b) Libermann Burchard’s test: Extracts were treated with chloroform and filtered. The filtrates were treated with few drops of acetic anhydride, boiled and cooled. Conc. Sulphuric acid was added. Formation of brown ring at the junction indicates the presence of phytosterols. |
| |
|
6. Detection of phenols
|
| |
| Ferric Chloride Test: Extracts were treated with 3-4 drops of ferric chloride solution. Formation of bluish black colour indicates the presence of phenols. |
| |
|
7. Detection of tannins
|
| |
| Gelatin Test: To the extract, 1% gelatin solution containing sodium chloride was added. Formation of white precipitate indicates the presence of tannins. |
| |
|
8. Detection of flavonoids
|
| |
| a) Alkaline Reagent Test: Extracts were treated with 4-5 drops of sodium hydroxide solution. Formation of intense yellow colour, which becomes colourless on addition of dilute acid, indicates the presence of flavonoids. |
| |
| b) Lead acetate Test: Extracts were treated with 4-5 drops of lead acetate solution. Formation of yellow colour precipitate indicates the presence of flavonoids. |
| |
|
9. Detection of proteins and aminoacids
|
| |
| a) Xanthoproteic Test: The extracts were treated with 4-5 drops of conc. Nitric acid. Formation of yellow colour indicates the presence of proteins. |
| |
| b) Ninhydrin Test: To the extract, 0.25% w/v ninhydrin reagent was added and boiled for few minutes. Formation of blue colour indicates the presence of amino acid. |
| |
|
10. Detection of diterpenes
|
| |
| Copper acetate Test: Extracts were dissolved in water and treated with 3-4 drops of copper acetate solution. Formation of emerald green colour indicates the presence of diterpenes [12, 13]. |
| |
|
Anthelmintic Assay
|
| |
| The assay was performed on adult earthworm, Eisenia foetida due to its anatomical and physiological resemblance with the intestinal roundworm parasite of human beings. Because of easy availability, earthworms have been used extensively for the preliminary in vitro evaluation of anthelmintic compounds in vitro [14]. 100 ml formulations containing four different concentrations, each of aqueous and ethanolic extract of its various fractions (10, 25, 50 and 100 mg/ml in distilled water) were prepared and six worms (same type) were placed in it. Time for paralysis was noted when no movement of any sort could be observed except when the worms were shaken vigorously. Time for death of worms were recorded after ascertaining that the worms neither moved when shaken vigorously nor when dipped in warm water (50 °C). Levamisole (0.55 mg/ml) was used as reference standard. |
| |
|
Statistical analysis
|
| |
| Data were analyzed using one way factorial ANOVA tests followed by Dunnett’s t -tests on each group. P values under 0.01 were considered highly significant (shown as **). |
| |
RESULT
|
| |
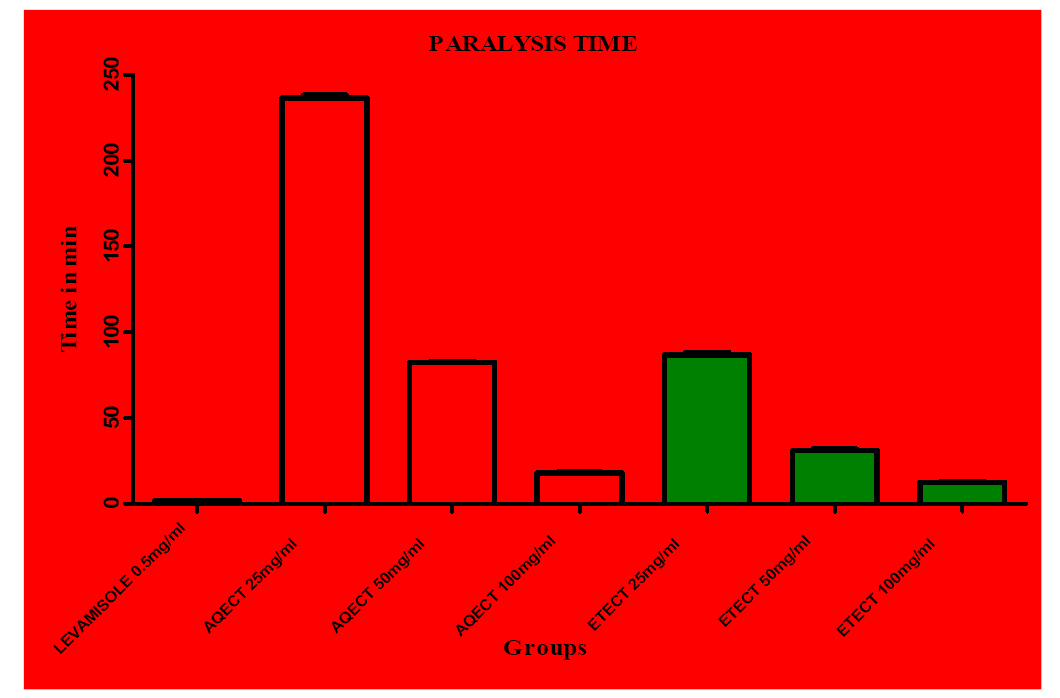
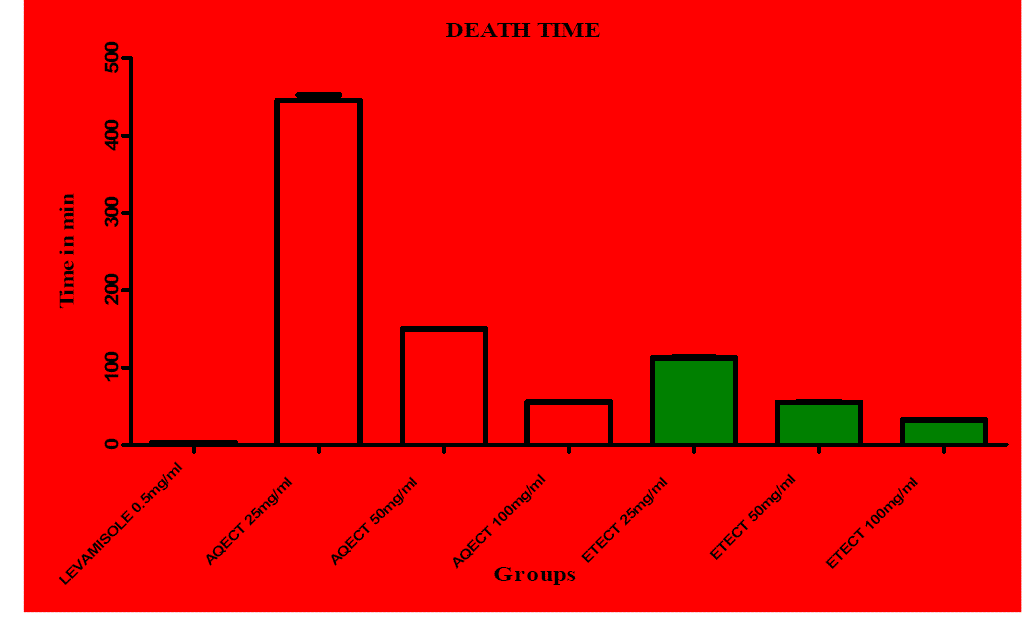
| Preliminary phytochemical screening of Clitoria ternatea extract revealed the presence of proteins, saponins, steroids, carbohydrates, alkaloids, tannins, glycosides, flavonoids and phenols (Table 1). The anthelmintic activity of ethanolic extract of Clitoria ternatea (ETECT) was more potent than aqueous extract of Clitoria ternatea (AQECT) was comparable with that of standard drugs levamisole. At the concentration of 100 mg/ml both the ethanolic and the aqueous extracts showed very significant activities as compared to the standard drug levamisole (0.55 mg/ml), In case of aqueous extract the time of paralysis and death time was observed as 18 ± 1.57 and 53.33 ± 0.33 and in case of ethanolic extracts 12.33 ± 0.80 and 32.33 ± 0.71 respectively. The extracts of Clitoria ternatea produced a significant anthelmintic activity in dose dependent manner as shown in (Table 2) and the graph shows (Figure 1 and Figure 2) Anthelmintic potential of both the extract with respect to levamisole. |
| |
DISCUSSION
|
| |
| Earthworms are invertebrates composed of many segments. They don’t have bones and move by contracting and relaxing the body segments in sequence. Earthworms have the ability to move by ciliary movement. The outer layer of the earthworm is a mucilaginous layer and composed of complex polysaccharides. This layer being slimy, enables the earthworm to move freely. Any damage to the mucopolysaccharide membrane will expose the outer layer and this restricts its movement and can cause paralysis. This action may lead to the death of the worm by causing damage to the mucopolysaccharide layer [15]. |
| |
| All anthelmintics essentially kill worms by either starving them to death or paralyzing them because worms have no means of storing energy, they must eat almost continuously to meet their metabolic needs. Any disruption in this process results in energy depletion. Interfering with feeding for 24 hours or less is sufficient to kill most adult parasites. Parasites will also die if they become paralyzed and temporarily lose their ability to maintain their position in the gut [16]. |
| |
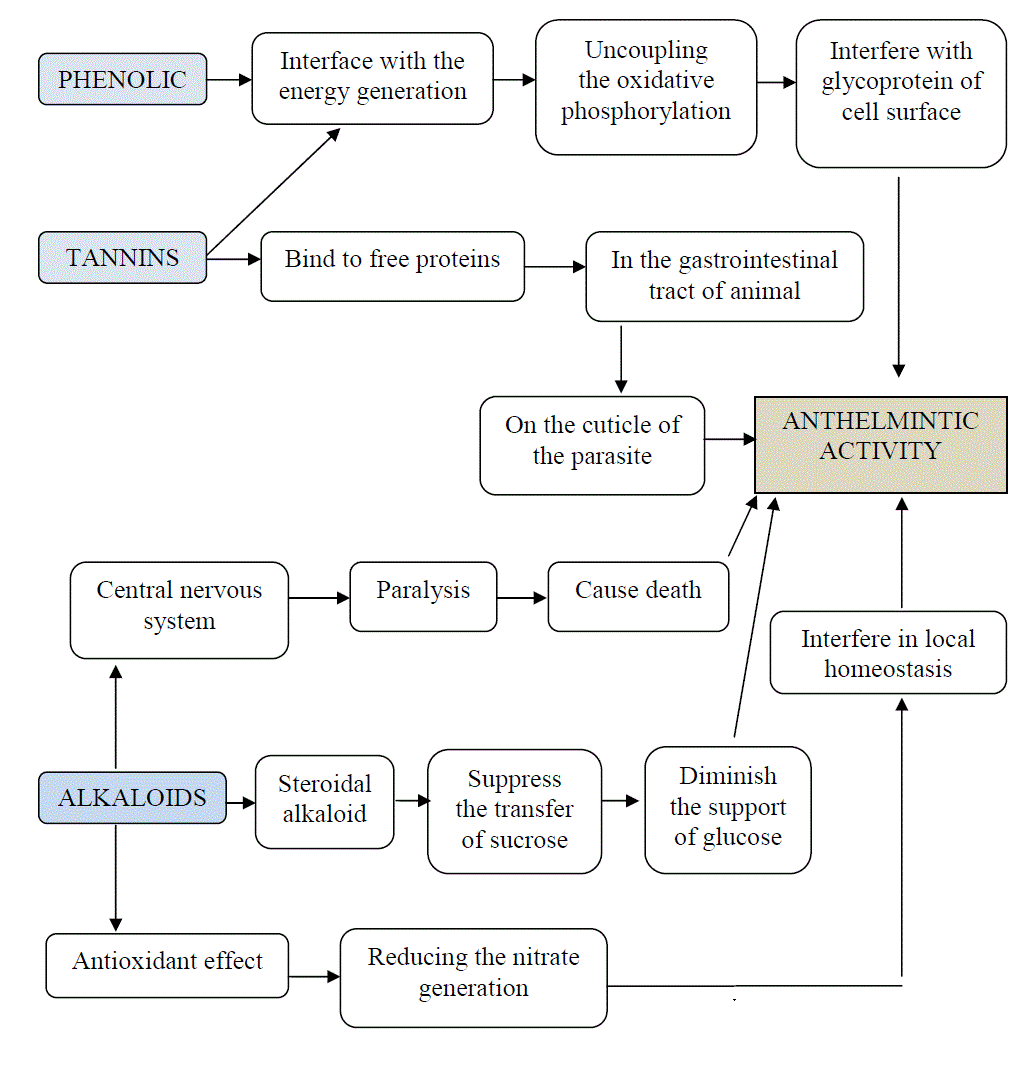
| Preliminary phytochemical screening of Clitoria ternatea extract revealed the presence of proteins, saponins, steroids, carbohydrates, alkaloids, tannins, glycosides, flavonoids and phenols. Synthetic phenolic anthelmintics interfere with the energy generation in the helminth parasites by uncoupling the oxidative phosphorylation. Another possible mechanism of action is that they bind to free proteins in the gastrointestinal tract of the host animal or to glycoprotein on the cuticle of the parasite and causes death [17]. |
| |
| The possible mechanism of action of tannins may be: |
| |
| a. Interfere with energy generation by uncoupling oxidative phosphorylation |
| |
| b. They may interfere with glycoprotein of cell surface |
| |
| c. They can bind to free proteins in the gastrointestinal tract of host animal or glycoprotein on the cuticle of the parasite and cause death. [18]. |
| |
| Alkaloids may act on central nervous system and caused paralysis of the earthworm [19]. The effect would be due to presence of the steroidal alkaloid oligoglycosides which may suppress the transfer of sucrose from the stomach to the small intestine together with its antioxidant effect which is capable of reducing the nitrate generation which could interfere in local homeostasis which is essential for the development of helminthes [20]. |
| |
| Levamisole are nicotinic receptor agonists and elicit spastic muscle paralysis due to prolonged activation of the excitatory nicotinic acetylcholine (nACh) receptors on body wall muscle. [21]. |
| |
CONCLUSION
|
| |
| It can be concluded that active constituents responsible for anthelmintic activity are present in the aqueous and ethanolic extracts of leaves of Clitoria ternatea. Further work will emphasize the isolation and characterization of active principles responsible for anthelmintic activity of leaf extracts of Clitoria ternatea. |
| |
ACKNOWLEDGEMENT
|
| |
| The authors are thankful to Dr. Monica Gulati, Dean, Department of Pharmaceutical Sciences, Lovely Professional University, Phagwara (Punjab) for providing necessary facilities and cooperation during this research work. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |









