Keywords
Activ gutta percha points; Antimicrobial activity; E. faecalis; S. mutans
Introduction
The fundamental goal of root canal treatment is to eliminate bacteria from the root canal and prevent reinfection. Because of the complex anatomy of root canal systems biomechanical preparation procedures do not completely eliminate them. Enterococcus faecalis and Streptococcus mutans are the most commonly isolated microorganisms from infected root canals. E. faecalis is associated with persistent apical periodontitis and resists elimination from root canals [1]. Streptococcus species were reported to be one of the most relevant taxa in symptomatic apical periodontitis [2].
E. faecalis is found in 4 to 40% of primary endodontic infections [3]. Failed root canal treatment cases are nine times more likely to contain E. faecalis than primary endodontic infections [3].
S. mutans is another microorganism found in infected root canals associated with apical periodontitis [4]. S. mutans is relatively uncommon, it has been shown to be one of the most convenient microorganisms for use in the infected dentine model because of its ability to adapt to the laboratory setting, unlike strict anaerobic species [5].
Calcium hydroxide plays an important role in endodontics by its ability to induce hard tissue formation, moderate antibacterial action, and tissue dissolving capability [6]. Calcium hydroxide dressing may prevent root canal reinfection by interrupting nutrient supply to remaining bacteria [7].
Chlorhexidine has inhibitory effect on bacteria commonly found in endodontic infections acting against gram positive and gram negative microorganisms [8,9]. Its efficacy is based on the interaction between positive charge of the molecule and negatively charged phosphate groups on the bacterial cell wall. which allows chlorhexidine molecule to penetrate into bacteria with toxic effects [10].
In response to potential difficulties of conventional intra canal medicaments which were used in paste form and difficult to remove, sustained releasing devices were developed [11]. Such active gutta-percha points contain substances with antimicrobial activity. Calcium hydroxide and Chlorhexidine gutta-percha points are among them [11].
The purpose of this study is to determine the antibacterial effectiveness of calcium hydroxide and chlorhexidine gutta percha points against E. faecalis and S. mutans.
Materials and Methods
Eighteen extracted straight single rooted human maxillary canines with single canal were selected for this study. Roots with resorption, fractures or open apices were excluded from this study. Soft tissue and calculus were removed from root surfaces with hand instrumentation and all teeth were stored in sterile saline solution until used.
Preparation of tooth samples
The teeth were decoronated at cement enamel junction to provide easy access to the canal space and to obtain a constant reference point for all instruments (Figure 1). And roots of all specimens were standardized to length of 17 mm (Figure 2). The contents of the canals were removed with barbed broach Initial apical patency of canal was checked with 10k-file (Figure 3) and canals were instrumented upto size 40k-file (Figure 4). Subsequently canals were irrigated with 2.0 ml 2.5% sodium hypochlorite followed by 2.0 ml saline solution. Then apical seal was established with type II glass ionomer cement. External root surface of all specimens were coated with nail varnish to close dentinal tubules. And tooth samples were mounted on self-cure acrylic resin and are autoclave sterilized at 121°C for 15 minutes (Figure 5). And they were divided into two groups with sample size of nine for each Enterococcus faecalis group and streptococcus mutans group. And they were subdivided into Conventional gutta-percha group. Calcium hydroxide group and CHX group with sample size of three each.

Figure 1: Decoronation at cemento enamel junction.
Figure 2: Standardization of root lengths to 17 mm.
Figure 3: Initial apical patency with 10k file.

Figure 4: Master apical file of size 40k file.
Figure 5: Mounting of specimens on acrylic blocks.
Preparation of inoculum
All microbiological studies were conducted under aseptic conditions to prevent airborne bacterial contamination. E. faecalis and S. mutans stains were inoculated with the loop into peptone water and they were incubated for 24 hrs at 37°c under aerobic conditions till turbidity is obtained (Figures 6 and 7).

Figure 6: Peptone water with E. faecalis turbidity.

Figure 7: Peptone water with S. mutans turbidity.
Treatment of tooth samples
0.5 ml of bacterial suspension was inoculated into mounted tooth samples of respective groups (Figure 8) and conventional gutta percha, Calcium hydroxide, and CHX were inserted into respective groups (Figure 9). They were sealed coronally with GIC and the tooth samples were incubated at 37°C for 24 hrs.
Figure 8: Inoculation of bacterial suspension into tooth samples.

Figure 9: Insertion of gutta percha points into tooth samples.
Analysis of colony forming units
Gutta percha points were removed from respective groups and the gutta-percha points were washed with 1 ml of saline (0.9% sterile NaCl) solution each to recover as many bacteria as possible. Tooth samples were sectioned with disc and mandril (Figure 10) than dentin was scraped with spoon excavators along the canal walls and were inoculated into test tubes containing peptone water (Figure 11). Test tubes containing peptone water is incubated for 24 hrs at 37°C aerobically till turbidity is obtained. Than the inoculum of respective groups was inoculated into agar plates and incubated for 24 hrs at 37°C for colony forming units.
Figure 10: Sectioning with disc mandrel.

Figure 11: Dentin scraping with spoon excavator.
Statistical Analysis
Data was analysed using SPSS version 22, Descriptive statistics Friedman’s test for intragroup comparison at various time periods. One way Anova for intergroup comparison at different time periods. Overall two way Anova for comparison between the groups and the bacteria (Table 1 and Graph 1).
| Group |
1 hour |
3 hours |
6 hours |
p value of Bacteria A at various time periods |
p value of Bacteria B at various time periods |
| Enterococcus |
Streptococos |
Enterococcus |
Streptococos |
Enterococcus |
Streptococos |
| Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
Mean |
SD |
| Chlorhexidine |
7.167 |
0.3055 |
7.733 |
0.3215 |
5.800 |
0.4359 |
5.533 |
0.8386 |
3.100 |
0.2000 |
3.200 |
0.2000 |
0.05* |
0.05* |
| calcium hydroxide |
7.633 |
0.3512 |
7.900 |
0.3606 |
7.167 |
0.3055 |
7.267 |
0.5508 |
6.600 |
0.4359 |
7.167 |
0.3055 |
0.05* |
0.097 NS |
| Conventional |
8.067 |
0.2082 |
8.133 |
0.3512 |
8.100 |
0.3606 |
8.067 |
0.5508 |
8.000 |
0.3606 |
7.933 |
0.3512 |
1 NS |
0.717 NS |
Intergroup comparison
p value |
0.027* |
0.001** |
<0.001** |
0.416 NS |
0.009** |
<0.001** |
<0.001** |
<0.001**# |
p value <0.05 is considered as statistically significant; * Statistically Significant (p<0.05) **; Statistically Highly Significant (p<0.01); NS - Not Signifcant (p>0.05); # - overall two way anova p value (comparisons between the groups and the bacteria)
Table 1: Intragroup comparison of various clinical parameters.
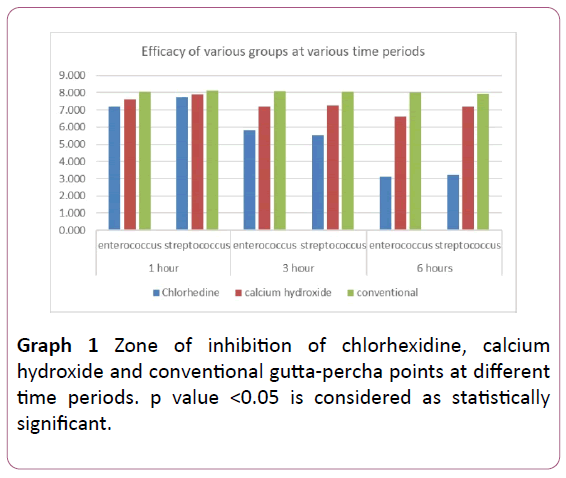
Graph 1: Zone of inhibition of chlorhexidine, calcium hydroxide and conventional gutta-percha points at different time periods. p value <0.05 is considered as statistically significant.
X-axis represents time intervals and Y axis represents zone of inhibition of E. faecalis and S. mutans with chlorhexidine gutta percha points, calcium hydroxide gutta percha points and conventional gutta percha points.
Results
The colony forming units of E. faecalis and S. mutans after treatment with Ca(OH)2, CHX and conventional gutta percha points were shown (Figures 12-17).
Figure 12: Conventional gutta percha group showing colony forming units at 1 hr, 3 hrs and 6 hrs.

Figure 13: Calcium hydroxide group showing colony forming units at 1 hr, 3 hrs and 6 hrs.

Figure 14: Chlorhexidine group showing colony forming units at 1 hr, 3 hrs and 6 hrs.
Figure 15: Conventional gutta percha group showing colony forming units at 1 hr, 3 hrs and 6 hrs.
Figure 16: Calcium hydroxide group showing colony forming units at 1 hr, 3 hrs and 6 hrs.
Figure 17: Chlorhexidine gutta percha points showing colony forming units at 1 hr, 3 hrs and 6 hrs.
There is significance decrease in colony forming units with increase in time interval upto 6 hrs in Ca(OH)2 and CHX groups.
Table 1 shows the mean and SD of the zone of inhibition at various time periods.
Intragroup comparison at various time periods was done using Friedman test showed there is a statistically significant differences present between the mean values at various time periods in chlorhexidine group calcium hydroxide and conventional group which showed that chlorhexidine is more effective for Enterococcus.
Intragroup comparison at various time periods was done using Friedman test showed there are statistically significant differences present between the mean values of mean zone of inhibition at various time periods in chlorhexidine calcium hydroxide & conventional group for streptococcus which showed that chlorhexidine is more effective for streptococcus.
Intergroup comparison is done by one way Anova showed statistically significant difference at all the time periods in both bacteria.
Overall two way Anova showed that there is statistically significant difference in mean zones of inhibition at various time periods in both the bacteria and the chlorhexidine is more effective than others(p <0.001).
Discussion
Endodontic therapy is based on nonspecific elimination of intra radicular microorganisms. Many studies have shown the importance of intracanal dressings for elimination of bacteria that cannot eliminated by biomechanical preparation. Several antimicrobial agents have been tested for their ability to eliminate E. faecalis and S. mutans from the root canal system. These include both irrigants, such as sodium hypochlorite, hydrogen peroxide, chlorhexidine digluconate and iodine compounds, as well as interappointment dressings, such as calcium hydroxide, chlorhexidine gluconate, camphorated phenol and mixed antibiotic-steroid combinations. This study investigated the antimicrobial potential of three different medicaments i.e., calcium hydroxide, chlorhexidine and conventional gutta-percha points against E. faecalis and S. mutans.
This study mimicked clinical conditions by using human teeth instead of bovine teeth. E. faecalis and S. mutans were chosen as test microorganisms in this study because they are most common dental pathogens [12]. E. faecalis has been shown to exhibit widespread genetic polymorphisms. It possesses serine protease, gelatinase, and collagen-binding protein (Ace), which help it bind to dentin.
E. faecalis is small enough to proficiently invade and live within dentinal tubules. It has the capacity to endure prolonged periods of starvation until an adequate nutritional supply becomes available. Once available, the starved cells are able to recover by utilizing serum as a nutritional source. Serum, which originates from alveolar bone and the periodontal ligament, also helps E. faecalis bind to type I collagen [3].
S. mutans has been detected in root canal infections and was also reported to be a strong biofilm producer [4,13], helping the bacteria to adapt and persist in root canals. The results of this study clearly indicated that Ca(OH)2 was not sufficient for the complete elimination of S.mutants from root canals.
In this study dentin chips were directly collected into peptone water and not in saline or transport medium which helps the growth of E. faecalis and S. mutans this method was modification of previous study done in which they used RTF or VMGAIII as medium [14].
This study demonstrated that the number of CFU’s were less in chlorhexidine impregnated gutta percha points than Ca(OH)2 guttapercha points. This relative inefficacy of Ca(OH)2 against E. faecalis was consistent with previous studies [15-17]. Ca(OH)2 was effective in the superficial dentine compared with the deeper layers [12]. The reason for this result was reported to be the superficial exposition of microorganisms to lethal levels of hydroxyl ions only at the tubule orifice [7].
One mechanism that can explain the in vivo antibacterial activity of Ca(OH)2 is to absorb CO2 in the root canals which is essential for bacteria such as Capnocytophagia, Eikenella and Actinomyces sps, and is provided by the bacteria such as Bacteriods, Fusobacterium, Porphyromonas [18,19].
Chlorhexidine in gel formulations have important properties such as low cytotoxicity to periapical tissues viscosity that keeps the active agent in contact with root canal walls, dentinal tubules and water solubility [20,21]. But in the present investigation chlorhexidine impregnated gutta percha points were used which showed antibacterial efficacy with increased time intervals upto 6 hrs against E. faecalis and S. mutans. This result agrees with those of others eventhough those studies utilized chlorhexidine in liquid or gel forms at different concentrations [22-24].
Hence the results of present investigation shows that chlorhexidine impregnated gutta-percha points were more effective against E. faecalis and S. mutans when compared with calcium hydroxide and conventional gutta percha points.
Conclusion
Within the limitation of this in vitro study it can be concluded that there is significant difference in the antimicrobial activity of Calcium hydroxide points and Chlorhexidine impregnated gutta percha points (Activ points) against Enterococcus faecalis and Streptococcus mutans. Chlorhexidine impregnated gutta perch points are more effective than Calcium hydroxide gutta percha points and conventional gutta percha points.
23975
References
- Dahlen G, Samuelsson W, Molander A, Reit C (2000) Identification and antimicrobial susceptibility of enterococci isolated from the root canal. Oral Microbiol Immunol 15(5): 309-312.
- Rocas IN, Siqueira JF Jr, Debelian GJ (2011) Analysis of symptomatic and asymptomatic primary root canal infections in adult Norwegian patients. J Endod 37(9): 1206-1212.
- Stuart CH, Schwartz SA, Beeson TJ, Owatz CB (2006) Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J Endod 32(2): 93-98.
- Gomes BP, Pinheiro ET, Gade Neto CR, Sousa EL, Ferraz CC, et al. (2004) Microbiological examination of infected dental root canals. Oral Microbiol Immunol 19(2): 71-76.
- Kreth J, Kim D, Nguyen M, Hsiao G, Mito R, et al. (2008) The antimicrobial effect of silver ion impregnation into endodontic sealer against Streptococcus mutans. Open Dent J 2: 18-23.
- Nerwich A, Figdor D, Messer HH (1993) PH changes in root dentin over a 4-week period following root canal dressing with calcium hydroxide. J Endod 19(6): 302-306.
- Siqueira JF Jr, Lopes HP (1999) Mechanisms of antimicrobial activity of calcium hydroxide:A critical review. Int Endod J 32(5): 361-369.
- Cervone F Tronstad L, Hammond B (1990) Antimicrobial effect of chlorhexidine in a controlled release delivery system. Endod Dent Traumatol 6(1): 33-36.
- Waler SM (1990) Further in vivo studies on the plaque inhibiting effect of chlorhexidine and its binding mechanisms. Scand J Dent Res 98(5): 422-427.
- Lindskog S, Pierce AM, Blomlof L (1998) Chlorhexidine as a root canal medicament for treating inflammatory lesions in the periodontal space. Endod Dent Traumatol 14(4): 186-190.
- Heling I, Sommer M, Steinberg D, Friedman M, Sela MN (1992) Microbiological evaluation of the efficacy of chlorhexidine in a sustained-release device for dentine sterilization. Int Endod J 25(1): 15-9.
- Lui JN, Sae-Lim V, Song KP, Chen NN (2004) In vitro antimicrobial effect of chlorhexidine-impregnated gutta percha points on Enterococcus faecalis. Int Endod J 37(2): 105-113.
- Jiang LM, Hoogenkamp MA, Van der Slugs LW, Wesselink PR, Crielaard W, et al. (2011) Metabolism assay for root canal disinfectant evaluation on dual-species biofilms. J Endod 37(1): 31-35.
- Haapasalo HK, Siren EK, Waltimo TMT, Orstavik D, Haapasalo MPP (2000) Inactivation of local root canal medicaments by dentine: An in vitro study. Int Endod Journal 33(2): 126 -131.
- Ercan E, Dalli M, Dulgergil CT (2006) In vitro assessment of the effectiveness of chlorhexidine gel and calcium hydroxide paste with chlorhexidine against Enterococcus faecalis and Candida albicans. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102(2): 27-31
- Delgado RJ, Gasparoto TH, Sipert CR, Pinheiro CR, Moraes IG, et al. (2010) Antimicrobial effects of calcium hydroxide and chlorhexidine on Enterococcus faecalis. J Endod 36(8): 1389-1393.
- Kandaswamy D, Venkateshbabu N, Gogulnath D, Kindo AJ (2010) Dentinal tubule disinfection with 2% chlorhexidine gel, propolis, morinda citrifolia juice, 2% povidone iodine, and calcium hydroxide. Int Endod J 43(5): 419-23.
- Kontakiotis E, Nakou M, Georgopoulou M (1995) In vitro study of the indirect action of calcium hydroxide on the anaerobic flora of the root canal. Int Endod J 28(6): 285-289.
- Sundqvist G (1992) Ecology of the root canal flora. J Endod 18(9): 427-430.
- Greenstein G, Berman C, Jaffin P (1986) Chlorhexidine an adjunct to periodontal therapy. J Periodontol 57(6): 370-376.
- Ferraz CCR, Gomes BPFA, Zaia AA, Teixeira FB, Souza-Filho FJ (2001) In vitro assessment of antimicrobial action and the mechanical ability or chlorhexidine gel as an endodontic irrigant. J Endod 27(7): 452-455.
- Siqueira JF, Uzeda M (1996) Disinfection by calcium hydroxide pastes of dentinal tubules infected with two obligate and one facultative anaerobic bacteria. J Endod 22(12): 674-676.
- Haapasalo HK, Siren EK, Waltimo TMT, Orstavik D, Haapasalo MPP (2000) Inactivation of local root canal medicaments by dentine: An in vitro study. Int Endod J 33(2): 126-131.
- Komorowski Regrade H, Wu XY, Friedman S (2000) Antimicrobial substantivity of chlorhexidine treated bovine root dentine. J Endod 26(6): 315-317.
























