Keywords
Complete revascularization; Culprit-only revascularization; Primary PCI; MACE
Introduction
Acute myocardial infarction (AMI) has been reported to be the leading cause of death in patients hospitalized for cardiovascular disease in industrial countries [1,2]. Among the various treatment approaches, primary percutaneous coronary intervention (p-PCI) is considered as the treatment of choice for patients presenting with ST-segment elevation myocardial infarction (STEMI), when it can be performed expeditiously by an experienced team [3-5]. This strategy has been reported to be superior to thrombolytic therapy in improving morbidity and mortality [6-8]. The goal of this treatment approach is the restoration of flow within 90 minutes of presentation to a PCI-equipped centre [9,10].
The prevalence of multi-vessel disease (MVD) has been reported to be 40-65% in patients with AMI undergoing p-PCI [11-14]. This finding suggests that a significant proportion of these patients have an increased risk of death and adverse outcomes even after receiving reperfusion therapy through thrombolysis or p-PCI for infarct-related artery (IRA) [12]. However, the prognostic impact of revascularization for non-IRA in patients with MVD after p-PCI on clinical outcomes has not been fully investigated [12,15-18].
Identification of optimal strategies for treating these patients is the subject of considerable interest and controversy. Treatment strategies vary widely from an aggressive approach, which treats all significant lesions in the acute phase of p-PCI, to a conservative approach with p-PCI of only the IRA and subsequent medical therapy unless recurrent ischemia occurs. Other treatment strategies include staged procedures in which the IRA is treated acutely and other lesions are treated later during the hospital stay or within the first month following hospital discharge.
Aim of the Work
The aim of the study is to compare p-PCI for culprit vessel only vs complete revascularization (CR) for all coronary tree in patients presenting with STEMI and having multi-vessel disease. Points of interest were LVEF, contrast induced nephropathy in addition to MACE during hospital stay and six months follow up.
Patients and Methods
A total of 130 pts with acute STEMI, who were amenable to primary coronary intervention, were admitted to the critical care department at the Cairo University October 2009-July 2011. Of them, 55 pts had multi-vessel coronary artery disease, and 40 of the 55 pts met our inclusion criteria. The selected participants were blindly randomized into two groups after signing an informed consent; Group A: complete coronary revascularisation during p-PCI and Group B: culprit-only revascularisation during p-PCI.
Inclusion criteria
Patients with acute STEMI and MVD were considered for our study. Those patients with cardiogenic shock, single vessel disease, left main disease (≥ 50% diameter stenosis), previous bypass surgery (CABG), severe valvular heart disease, in-stent restenosis and any contraindication to primary angioplasty were excluded from the study. All the patients were subjected to clinical examination and complete laboratory analysis including cardiac enzymes. Echocardiography was performed on admission, discharge and 6 months later.
All the patients received 150 mg aspirin, 600 mg clopidogrel, statins and heparin infusions. Other drugs such as IV nitroglycerin, ACE inhibitors, B-blockers, Ca-channel blockers, antiarrhythmics, vasopressor, IIb/IIIa antagonist and inotropes were given when indicated. The patients were subjected to coronary angiography and PCI. The follow-up visits were scheduled at 1 and 6 months. LVEF was assessed twice: first during hospital stay and the second at 6 months follow-up. Lesions were assessed by QCA by two operators and were classified as critical if >70% stenosis.
Contrast-induced Nephropathy (CIN)
CIN was defined as an elevation of serum creatinine (Scr) of more than 25% or >0.5 mg/dl (44 μmol/l) from baseline within 48 h after excluding other factors that may cause nephropathy, such as nephrotoxins, hypotension, urinary obstruction or atheromatous emboli [19].
Statistical analysis
Data were collected and coded prior to analysis using the professional Statistical Package for Social Science (SPSS 12). All data were expressed as mean+standard deviation. The Student’s t-test (unpaired) was performed after checking the normality for all continuous data. Mann-Whitney U test was used when the value of SD was violated. The chi-square test was performed for all categorical data to test for the presence of an association. For small sample size, Fisher’s exact test was performed. Multivariate regression analysis was performed for predictors of LVEF. A P-value <0.05 was considered to be significant.
Results
Baseline clinical and demographic data regarding age, sex, risk factors & previous history of IHD were found to be comparable in both groups (Table 1).
| Parameters |
Group A |
Group B |
P |
| Males |
15 (75%) |
18 (90%) |
0.21 |
| Females |
5 (25%) |
2 (10%) |
| Age |
54.1+10.26 |
56.25+8.1 |
0.46 |
| Diabetes |
10 (50%) |
9 (45%) |
0.75 |
| Hypertension |
9 (45%) |
9 (45%) |
1 |
| Dyslipidemia |
11 (55%) |
10 (50%) |
0.75 |
| Smoking |
15 (75%) |
16 (80%) |
0.7 |
| Family history |
6 (30%) |
4 (20%) |
0.46 |
| Previous history of IHD |
3 (15%) |
5 (25%) |
0.3 |
| MAP (mmHg) |
86.1 ± 12.6 |
88.3 17 |
0.64 |
| HR (bpm) |
86 ± 14.6 |
82 ± 16.2 |
0.41 |
| Site of MI by ECG |
|
| Anterior |
11 (55%) |
8 (40%) |
0.62 |
| Inferior |
8 (40%) |
11 (55%) |
| Both |
1 (5%) |
1 (5%) |
| Killip class. |
|
| Class I |
15 (75%) |
17 (85%) |
0.34 |
| Class II |
3 (15%) |
2 (10%) |
| Class III |
2 (10%) |
1 (5%) |
| Class IV |
0 (0%) |
0 (0%) |
| D to B time(mins) |
94 ± 13.6 |
93 ± 13.4 |
0.86 |
| Hospital stay(days) |
5.4 ± .76 |
5.5 ± 1.19 |
0.75 |
| Laboratory data |
| Ck1 (u/L) |
266.6 ± 281 |
261.0 ± 234.9 |
0.74 |
| CK2 |
2317.0 ± 1732.7 |
2129.0 ± 1421.7 |
0.95 |
| CK3 |
1757.5 ± 1347.1 |
1804.0 ± 779.9 |
0.55 |
| CKMB1(u/L) |
28.0 ± 35.4 |
24.6 ± 27.3 |
0.56 |
| CKMB2 |
181.4 ± 129.9 |
120.9 ± 76.7 |
0.27 |
| CK MB3 |
106.5 ± 81.7 |
81.1 ± 50.8 |
0.44 |
| Hb1(gm/dL) |
14.0 ± 1.8 |
13.6 ± 1.3 |
0.43 |
| Hb2 |
12.9 ± 1.6 |
12.8 ± 1.4 |
0.82 |
| CREAT.1 (mg/dl) |
1.05 ± 0.3 |
1.03 ± .32 |
0.75 |
| CREAT.2 |
1.1 ± 0.39 |
1.13 ± .39 |
0.9 |
| INR |
1.07 ± 0.16 |
1.13 ± .15 |
0.22 |
| Medications data |
| ACEI |
18 (90%) |
17 (85%) |
0.63 |
| BB |
17 (85%) |
18 (90%) |
0.63 |
| Copidogrel |
20 (100%) |
20 (100%) |
|
| Statins |
20 (100%) |
20 (100%) |
|
| Inotropes |
1 (5%) |
2 (10%) |
0.54 |
| GPIIb/IIIa |
20 (100%) |
20 (100%) |
|
IHD: Ischemic Heart Disease; MAP: Mean Arterial Pressure; HR: Heart Rate; MI: Myocardial Infarction; D to B: Door to balloon; CK: Creatine Kinase; Hb: Hemoglobin; CREAT: Creatinine; INR: International Normalized Ration; BB: Beta Blockers; ACE: Angiotension Converting Enzyme Inhibitor
Table 1 Demographic, clinical data, laboratory and medical data of the two groups.
Interventional data
In group A, the IRA was observed to be left anterior descending coronary artery (LAD) in 10 patients (50%), right coronary artery (RCA) in 5 patients (25%), and left circumflex artery and its branches in 5 patients (25%). Two-vessel disease was observed in 18 patients (90%) while the three-vessel disease was found in 2 patients (10%). However, in group B, the IRA was observed to be LAD in 9 patients (45%), RCA in 9 patients (45%), and Lt Cx in 2 patients (10%). Two-vessel disease was seen in 16 patients (80%) while the three-vessel disease was found in 4 patients (20%). The lesions types were found to be comparable between the two groups in the three major vessels (LAD, CX and RCA) (Table 2).
| Parameters |
Group A |
Group B |
P value |
| Angiographic data |
| Infarct related artery |
|
| LAD |
10 (50%) |
9 (45%) |
0.37 |
| Lt CX |
1 (5%) |
2 (10%) |
| RCA |
9 (45%) |
9 (9%) |
| Diseased vessels |
|
| 2 vessels |
18 (90%) |
16 (80%) |
0.13 |
| 3 vessels |
2 (10%) |
4 (20%) |
| LAD lesions No. |
17 |
16 |
|
| Types A |
0 |
1 |
0.57 |
| B |
10 |
9 |
| C |
7 |
6 |
| CX lesions No. |
11 |
14 |
|
| Types A |
0 |
1 |
0.49 |
| B |
9 |
12 |
| C |
2 |
1 |
| RCA lesions No. |
14 |
14 |
|
| Types A |
2 |
1 |
0.47 |
| B |
8 |
11 |
| C |
4 |
2 |
| PCI data |
| Total stents |
42 |
21 |
<0.0001 |
| BMS |
36 |
18 |
|
| DES |
6 |
3 |
0.92 |
| Contrast dose |
300.0 ±72.5 |
265.0 ±48.9 |
0.08 |
| Procedural duration |
69.7±12.5 |
52.0 ±11.3 |
<0.0001 |
| Procedural success |
20 (100%) |
20 (100%) |
|
| Angiographic complications |
1 (5%) |
2 (10%) |
0.5 |
Table 2: Angiographic and PCI data.
Door-to-balloon time
The mean door-to-balloon time was comparable between the two groups (Group A, 94 min ± 13.6; Group B, 93 min ± 13.9 with a P-value 0.86). Patients with a door-to-balloon time less than 90 minutes had better LVEF than patients with a door-to-balloon time of more than 90 minutes (57.1 ± 6.3 vs. 50.5 ± 7.3; P-value, 0.005). A negative correlation was observed between LVEF and door-to-balloon (D to B) time (r = −0.63, P-value < 0.001) (Figure 1).
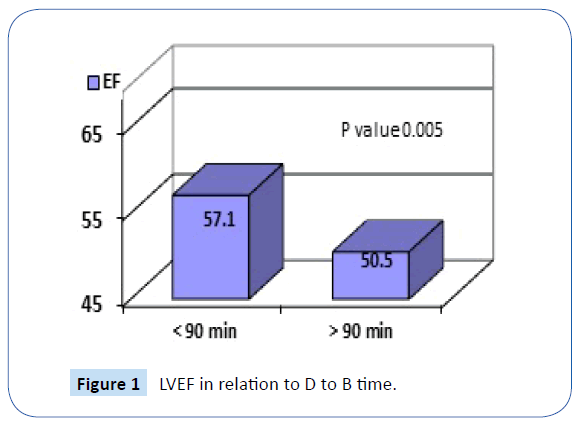
Figure 1: LVEF in relation to D to B time.
Contrast dose
The contrast dose used during the primary intervention was higher in group A, but it was not statistically significant (300 ± 72.5 ml vs. 265 ± 48.9 ml; P-value 0.08).
Procedure duration
The duration of intervention was significantly higher in group A in relation to group B (69.7 ± 12.5 min vs. 52 ± 11.3 min; P-value<0.0001)
Procedure success and complications
The intervention was successful in all patients of group A and 19 patients of group B (TIMI III and residual stenosis <30%). The incidence of complications was not significant between both the groups (Figure 2). In group A, distal embolisation was observed in 1 patient, serious arrhythmia in 2 patients and hypotension necessitating inotropic support was found in 2 patients. In group B, distal embolisations were observed in 2 patients and serious arrhythmia in 1 patient.
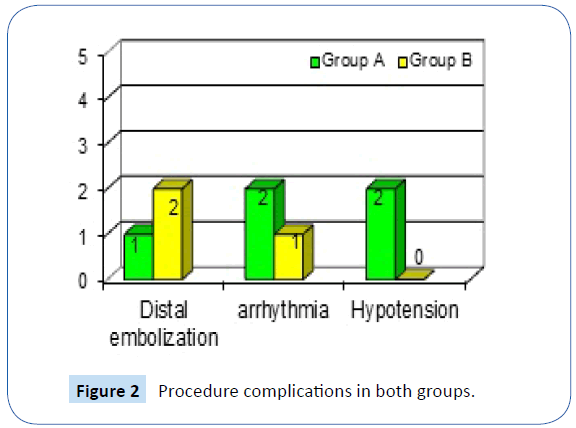
Figure 2: Procedure complications in both groups.
In-Hospital MACE
No MACE was observed in group A while one case of MACE (death) was found in group B (P=0.31). This patient had TIMI II flow after PCI, and the cause death was Ventricular Fibrillation (Figure 3).
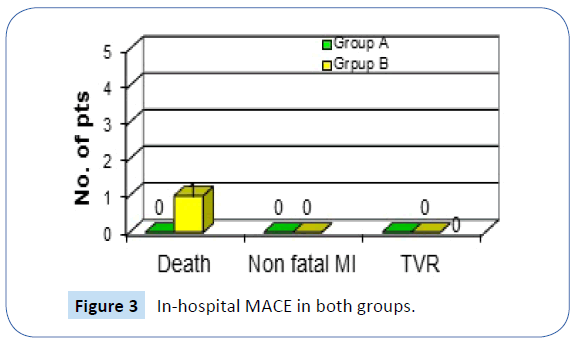
Figure 3: In-hospital MACE in both groups.
Hospital stay
The mean hospital stay (days) was 5.4 ± 0.7 in group A and 5.5 ± 1.19 in group B (P-value 0.75). The hospital stay was comparable between both groups, and there was no need for more stay in case of CR.
Vascular complications
There was no incidence of major bleeding or site access complications in both groups. Only one patient with minor bleeding was observed in group A (P-value 0.31).
Contrast-induced nephropathy (CIN)
CIN was observed in two patients of group A and one patient of group B (P-value 0.54). Thus, the incidence of CIN was comparable between both groups, indicating no added risk to the patient with an aggressive strategy of CR (Figure 4).
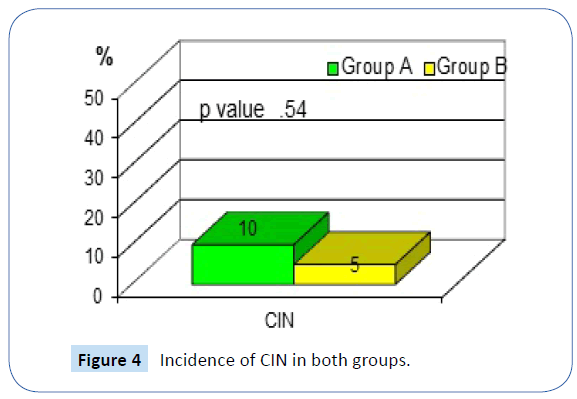
Figure 4: Incidence of CIN in both groups.
However, the subgroup analysis showed that CIN was statistically significant in patients with anterior myocardial infarctions (10.5% in patients with anterior MI while 0% in patients with inferior MI, P-value 0.03) (Figure 5).
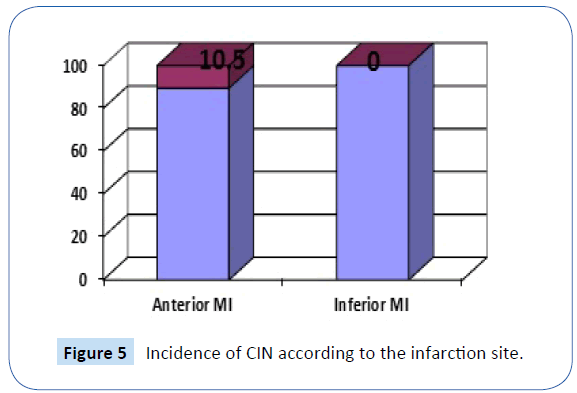
Figure 5: Incidence of CIN according to the infarction site.
MACE for 30 days (including in-hospital MACE)
There was no incidence of MACE in group A. However, in group B, there were two cases, one hospital death and the other nonfatal MI requiring reintervention in the same vessel territory. However, the analysis of the data for these patients showed that they stopped clopidogrel prematurely (P-value = 0.14).
MACE for 6 months and rehospitalisation
The incidence of MACE and rehospitalisation at 6 months were the same as at 1 month follow-up between both groups. Only one patient in group A needed reintervention. All patients in group B received intervention in the non-culprit vessels after 1 month from p-PCI.
Left Ventricular Ejection Fraction (LVEF) (6 months)
During hospital stay: The LVEF was 54.3 ± 9.1 in group A and 54.9 ± 5.2 in group B (P=0.81).
At 6 months follow-up:
• LVEF in group A increased significantly to 58.4 ± 6.2 (P=0.002).
• While in group B, it increased non-significantly to 55.7 ± 6.7 (P=0.55)
The increase in LVEF after 6 months was significant in patients with anterior MI (51.89 ± 7.5 » 55.16 ± 6.8, P-value 0.004) (Table 3). However, it was not significant in patients with inferior MI (57.68 ± 5.7 » 59.4 ± 5.6, P-value 0.36) (Figure 6 and 7).
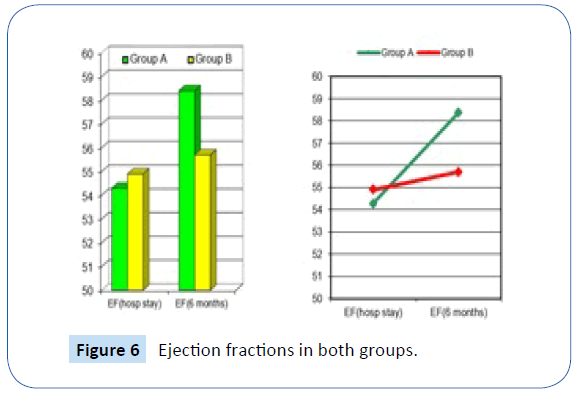
Figure 6: Ejection fractions in both groups.
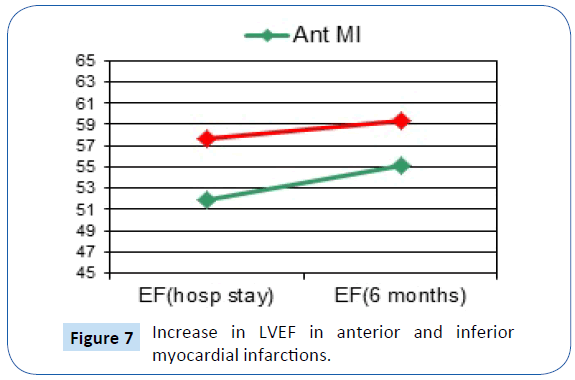
Figure 7: Increase in LVEF in anterior and inferior myocardial infarctions.
| Parameters |
Group A |
Group B |
| LVEF (hosp. stay) |
54.3 ± 9.1 |
54.9 ± 5.2 |
| LVEF (6 months) |
58.4 ± 6.2 |
55.7 ± 6.7 |
| P value |
0.002 |
0.55 |
Table 3 Comparison between LVEF in both groups.
Multivariate regression analysis for predictors of LVEF at 6 months showed that GROUPING (CR or COR) was found to be the only significant predictor for LVEF at 6 months (Table 4).
| Model |
P-VALUE |
| GROUP |
0.014 |
| DM |
0.59 |
| ECG |
0.6 |
| KILL Class |
0.17 |
| D to B time |
0.36 |
| Diseased vessel |
0.12 |
Table 4 Multivariate regression analysis for predictors of LVEF at 6 months.
Discussion
Our study has demonstrated that CR during the index admission in patients undergoing p-PCI for STEMI resulted in a better outcome rate at six months. This was clearly evident on the LV function after 6 months in group A (CR) (LVEF increased significantly from 54.3 ± 9.1 to 58.4 ± 6.2; P-value 0.002) compared to group B (COR) (it increased non-significantly from 54.9 ± 5.2 to 55.7 ± 6.7; P-value 0.55).
The PRIMA trial compared the two therapeutic approaches: 1-complete versus 2-stage percutaneous revascularization in patients with STEMI and MVD in relationship to the recovery of left ventricular systolic function. The principle finding of the study is that in patients randomly assigned to 1-stage percutaneous revascularization, the left ventricular ejection fraction (LVEF) recovers more rapidly and more significantly in comparison to the standard 2-stage procedure.
The 1-stage complete PCI led to a significant improvement of LVEF in AMI patients with MVD after 30 days, with a trend towards further improvement at 6-month follow-up as compared to the 2-stage approach [20].
The influence of MVD on the recovery of LV function was assessed by Ottervanger et al. [21] in 600 patients with AMI treated with p-PCI. They showed that despite the regained flow in the IRA, the presence of MVD was correlated with a lack of a significant improvement of LVEF.
The recovery of left ventricular function after complete multivessel one-stage PCI in patients with acute STEMI was assessed by Andrzej et al. [15] in 48 patients (group A) and two-stage PCI in 44 patients (group B). In group A, the absolute LVEF increase after 30 days was significantly higher in comparison to group B (P-value <0.01). A similar trend was observed after 180-day follow-up, and the difference was borderline significant (P-value 0.052). A significantly higher percentage of patients in group A reached the primary endpoint (increase in LVEF > 5%) compared to group B (44.7% vs. 32.4%, P-value 0.028).
Subgroup analysis suggested benefit of patients with anterior MI over other ACS. There was a trend towards an increase in LVEF % in group A, especially in patients presenting with anterior MI.
Patients with door-to-balloon time less than 90 minutes had better LVEF than patients with door-to-balloon time more than 90 minutes (57.1 ± 6.3 vs. 50.5 ± 7.3, P-value=0.005). This is in accordance with the international guidelines for the door-toballoon less than 90 minutes [10].
The contrast dose used during the primary intervention was higher in group A, but it was not statistically significant (300 ± 72.5 ml vs. 265 ± 48.9 ml, P-value 0.08).
A recent meta-analysis confirmed that MVD is commonly present among STEMI patients presenting for P-PCI and has a negative impact on 30-days mortality [22]. However, previous studies addressing the management of N-IRA lesions have produced conflicting results, as reflected in the 2013 American College of Cardiology Foundation/American Heart Association [23] and 2012 European Society of Cardiology guidelines (IIIC and IIb Class C, respectively). These recommendations were made on the basis of studies that differed in design and consisted of subgroups of randomized p-PCI trials or retrospective observational registries [24]. This suggested that in-hospital CR appeared to be associated with worse outcomes. The reason for a trend toward increased mortality with immediate complete PCI in the non-randomized retrospective registry studies is likely attributable to case selection.
One-stage revascularization is associated with higher contrast medium load and longer procedure time in the setting of the acute phase of STEMI, but no increased incidence of adverse events and complications were noted in long-term follow-up. Two-stage PCI is associated with additional vascular access, stress to the patient, and prolonged hospitalization that increased costs. The mean hospital stay was comparable in patients treated with 1-stage PCI.
The incidence of MACE in both groups was comparable during the hospital stay and at 1 and 6 months follow-up. Two cases of MACE was observed in group B while no MACE cases were observed in group A at 1 and 6 months follow-up (P-value 0.14).
The safety of aggressive strategy for CR is comparable for culpritonly strategy with respect to incidence of (1) CIN: two cases in group A and 1 case in group B (P-value 0.54); (2) vascular complications: no cases in group A and only one case in group B (P-value 0. 31).
Limitations
Case selection bias cannot be completely excluded, but due to the very nature of MVD clinical trial in p-PCI setting, it is not possible to randomize patients before the results of the angiogram are known. Due to our limited resources, neither intravascular ultrasound nor FFR was used to assess lesion severity. This should be further studied in future. Lastly, future studies should use more number of patients on a multicentre basis.
Conclusion
The study has demonstrated that CR of angiographically significant N-IRA lesions patients with STEMI treated by p-PCI resulted in improved clinical outcomes compared with the treatment of culprit lesion only.
20861
References
- Zijlstra F, de Boer MJ, Hoorntje JC, Reiffers S, Reiber JH, et al.(1993) A comparison of immediate coronary angioplasty with intravenous streptokinase in acute myocardial infarction. N Engl J Med328: 680-684.
- Grines CL, Browne KF, Marco J,Rothbaum D, Stone GW, et al. (1993) A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med328: 673-679.
- Gibbons RJ,Holmes DR,Reeder GS (1993) Immediate angioplasty compared with the administration of a thrombolytic agent followed by conservative treatment for myocardial infarction. The Mayo Coronary Care Unit and Catheterization Laboratory Groups. N Engl J Med328: 685-691.
- Widimsky´ P,Budesı´nsky´T, Vora´c D,Groch L, Zelízko M, et al.(2003) ‘PRAGUE’ Study Group Investigators. Long distancetransport for primary angioplasty vs. immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial-PRAGUE-2. Eur Heart J24:94-104.
- Andersen HR,Nielsen TT, Rasmussen K(2003) DANAMI-2 Investigators. A comparisonofcoronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med349:733-742.
- Keeley EC, Boura JA, Grines CL (2003) Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet361: 13-20.
- Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, et al. (2008) Management ofacute myocardial infarction in patients presenting with persistent ST segment elevation: The Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J29:2909-2945.
- Antman EM, Hand M,ArmstrongPW,Bates ER, Green LA, et al. (2007) focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol51:210-247.
- CardarelliF, BellasiA, Fang-Shu O, Raggi P (2009) Combined impact of age and estimated glomerular filtration rate on in-hospital mortality after percutaneous coronary intervention for acute myocardial infarction (from the American College of Cardiology National Cardiovascular Data Registry). Am J Cardiol103: 766-771.
- Lee JH, Park HS, Chae SC(2009) Predictors of six-month major adverse cardiac events in 30-day survivors after acute myocardial infarction (from the Korea Acute Myocardial Infarction Registry). Am J Cardiol104: 182-189.
- Rasoul S, Ottervanger JP, de Boer MJ, Dambrink JH, Hoorntje JC, et al. (2009) Predictors of 30-day and 1-year mortality after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Coron Artery Dis 20:415-421.
- Toma M, Buller CE, Westerhout CM, Fu Y, O'Neill WW, et al.(2010) Nonculprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: insights from the APEX-AMI trial.Eur Heart J31:1701-1707.
- Lemesle G, de Labriolle A, Bonello L(2009) Incidence, predictors, and outcome of new, subsequent lesions treated with percutaneous coronary intervention in patients presenting with myocardial infarction. Am J Cardiol103: 1189-1195.
- Jaski BE, Cohen JD, Trausch J, Marsh DG, Bail GR, et al. (1992) Outcome of urgent percutaneous transluminal coronary angioplasty in acute myocardial infarction: comparison of single-vessel versus multivessel coronary artery disease. Am Heart J124: 1427-1433.
- Park DW, Clare RM, Schulte PJ, Pieper KS, Shaw LK, et al. (2014) Extent, location, and clinical significance of non-infarct-related coronary artery disease among patients with ST-elevation myocardial infarction. JAMA 312: 2019-2027.
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, et al. (2013) ACCF/AHA guideline for the management of ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 61: 78-140.
- Steg PG, James SK, Atar D (2012) ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J33:2569-2619.
- Toma M, Buller CE, Westerhout CM, Fu Y, O'Neill WW, et al. (2010)Non-culprit coronary artery percutaneous coronary intervention during acute ST-segment elevation myocardial infarction: Insights from the APEX-AMI trial. Eur Heart J31: 1701-1707.
- Mohammed NMA, Mahfouz A, Achkar K, Rafie IM, Hajar R (2013) Contrast-induced nephropathy. Heart Views 14: 106-116.
- Andrzej O, Grzegorz A, Wojciech W, Dudek D, Dziewierz A, et al. (2004) The function of the left ventricle after complete multivessel one-stage percutaneous coronary intervention in patients with acute. J Invasive Cardiol16.
- Ottervanger JP, Van’t hof AW, Reiffers S,Hoorntje JC, Suryapranata H, et al. (2001) Long-term recovery of left ventricular function after primary angioplasty for acute myocardial infarction. Eur Heart J 22: 785-790.
- Elsasser A, Schlepper M, Klovekorn WP, Cai WJ, Zimmermann R, et al. (1997) Hibernating myocardium: An incomplete adaptation to ischemia. Circulation 96:2920-2931.
- Harold JG (2013) ACCF/AHA/SCAI 2013 update of the clinical competence statement on coronary artery interventional procedures. Circulation 128: 436-472.
- Zeymer U, Vogt A, Zahn R, Weber MA, Tebbe U, et al. (2004) Predictors of in hospital mortality in 1333 patients with acute myocardial infarction complicated by cardiogenic shock treated with primary percutaneous coronary intervention (PCI): Results of the primary PCI registry of the Arbeitsgemeinshaft Leitende Kardiologische Krankenhausarzte (ALKK).Eur Heart J 25: 322-328.













