Keywords
Biomarkers; Cardiac biomarkers; Acute myocardial infarction; High-sensitivity troponin; Troponin; Creatine kinase; Type 2 myocardial infarction; Reinfarction; Sensitivity; Specificity; Cerebrovascular accident
Abbreviations
ACS: Acute Coronary Syndrome; AMI: Acute Myocardial Infarction; ACC: American College of Cardiology; cTn: Cardiac Troponin; CVA: Cerebrovascular Accident; CRF: Chronic Renal Failure; CV: Coefficient of Variation; CT: Computed Tomography; CABG: Coronary Artery Bypass Grafting; CK-BB: Creatine Kinase Brain/Brain Isoform; CK-MB: Creatine Kinase Muscle/Brain Isoform; CK-MM: Creatine Kinase Muscle/Muscle Isoform; CK: Creatine Kinase; EKG: Electrocardiogram; ESC: European Society of Cardiology; hs-cTn: High-Sensitivity Cardiac Troponin; hs-Tn: High-Sensitivity Troponin; LoD: Limit of Detection; MRI: Magnetic Resonance Imaging; MI: Myocardial Infarction; NPV: Negative Predictive Value; PCI: Percutaneous Coronary Intervention; TIA: Transient Ischemic Attack; TnI: Troponin I; TnT: Troponin T; Tn: Troponin
Introduction
Biomarkers have been used to help diagnose acute myocardial infarction (AMI) and other acute disease states for more than 40 years. CK isoforms/subforms, myoglobin, and Tn assays have improved and have been the mainstays in rapid diagnosis of AMI. Plasma serum levels of cardiac Tn (cTn) I and T have supplanted CK isoforms as the biomarker of choice for the diagnosis of MI [1].However, the CK isoforms/subforms not only remain a necessary confirmation modality of the Tn based indication of MI but have the potential to become the gold standard in the early diagnosis of CVA. In this review we propose a contemporary algorithm using these cardiac specific biomarkers for patients presenting with chest pain and patients with disease states associated with elevated TnT/I serum levels. We summarize advances in the use of Tn and CK isoforms as diagnostic markers for various disease states.
Materials and Methods
We examined current assays used for cardiac biomarkers in AMI, skeletal muscle trauma, renal failure, sepsis, and CVA by searching the internet using key words and published medical journals. We searched major studies, meta-analyses, and societal recommendations for diagnosis and treatment of these disease states.We applied a novel algorithm that streamlines the use of these assays to rapidly diagnose these disease states with higher sensitivity and specificity in certain patient populations. We used reported statistics from these studies to elucidate the algorithm and recommendations.
Results
Current assays and associated limitations
The American College of Cardiology (ACC) and European Society of Cardiology (ESC) have redefined the diagnostic criteria for MI [2]. These societies define a raised value of cTn as a measurement exceeding the 99th percentile of the values obtained from a reference group [2]. They propose the acceptable imprecision of Tn measurement at the 99th percentile should be <10% of the coefficient of variation (CV) [3]. Concurrent measurements of CK muscle/brain (CK-MB) and myoglobin are also used as markers for MI.
Prior to the introduction of hs-Tn assays, commercial assays, in general, were incapable of achieving a 10% CV at the 99th percentile reference limit [4]. With hs-Tn assays, accurate differentiation between “minor” myocardial injury and analytical noise can be achieved [5]. In the past, obtaining a precise cTn assay was challenging and differentiation between “minor” MI and analytical noise was often impossible [4]. However, results obtained with next-generation cTn assays show there has been considerable improvement in the sensitivity (Table 1) [4-9].
| Troponin Assay |
Sensitivity |
Specificity Value |
Negative Predictive Value |
Positive Predictive Value |
| Percent (95% confidence interval) |
| Sensitive troponin assays |
| Abbott-Architect Troponin I |
| Limit of detection, 0.010 µg/liter |
94 (88-97) |
87 (84-89) |
98 (97-99) |
59 (52-66) |
| 99th percentile, 0.028 µg/liter |
86 (79-92) |
92 (90-94) |
97 (95-98) |
69 (61-76) |
10% coefficient of variation,
0.032 µg/liter |
85 (77-90) |
93 (90-95) |
97 (95-98) |
70 (62-78) |
| Roche-High Sensitive Troponin T |
| Limit of detection, 0.002 µg/llter |
100 (97-100) |
14 (12-18) |
100 (96-100) |
19 (16-23) |
| 99th percentile, 0.014 µg/llter* |
95 (90-98) |
80 (77-83) |
99 (97-100) |
50 (43-56) |
| Roche Troponin I |
| Limit of detection, 0.100 µg/liter |
92 (86-96) |
88 (86-91) |
98 (97-99) |
62 (55-69) |
| 99th percentile, 0.160 µg/liter |
84 (76-90) |
94 (91-95) |
97 (95-98) |
73 (65-80) |
10% coefficient of variation,
0.300 µg/liter |
75 (66-82) |
97 (95-98) |
95 (93-97) |
83 (75-89) |
| Siemens Troponin I Ultra |
| Limit of detection, 0.006 µg/liter |
97 (91-99) |
68 (64-72) |
99 (97-100) |
38 (32-44) |
| 99th percentile, 0.040 µg/liter* |
89 (82-94) |
92 (89-94) |
98 (96-99) |
68 (60-76) |
| Standard assay |
| Roche Troponin T 4th Generation |
| 99th percentile, unknown |
| Limit of detection. 0.010 µg/liter |
83 (76-90) |
93 (91-95) |
97 (95-98) |
72 (64-79) |
10% coefficient of variation,
0.035 µg/liter |
72 (64-80) |
97 (96-98) |
94 (92-96) |
85 (76-91) |
| The criterion of 10% coefficient of variation was fulfilled at the 99th percentile. |
Table 1: Sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) for various Tn assays [7]. Data is presented in the form: n% (95% confidence interval range). From The New England Journal of Medicine, Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, et al., Early diagnosis of myocardial infarction with sensitive cardiac troponin assays, 361, 858-867 Copyright © (2009) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Sensitivity and specificity of biomarkers
Tables 1 and 2 show the sensitivity and specificity of TnT, TnI, total CK, CK-MB, and CK subforms.
Tables 1 and 2 represent the sensitivity and specificity of Biomarkers.
| Biomarker |
Sensitivity |
Specificity |
Hours after
presentation |
0 |
1 |
2 |
3 |
6 |
12-24 |
0 |
1 |
2 |
3 |
6 |
12-24 |
Total CK
(170 U/L men;
35 U/L women)* |
29.6 |
29.6 |
33.3 |
- |
81.5 |
81.5 |
83.9 |
84.6 |
85.2 |
- |
87.9 |
89.3 |
CK-MBmass
(5 ng/mL)* |
25.9 |
44.4 |
51.9 |
- |
100.0 |
100.0 |
96.6 |
97.3 |
94.6 |
- |
94.6 |
97.3 |
CK-MBmass
(8 ng/mL)** |
- |
- |
- |
36.0 |
87.0 |
98.0 -99.0# |
- |
- |
- |
99.0 |
94.0 |
88.0- 91.0# |
CK-MBacitivty
(8 U/L)** |
- |
- |
- |
20.0 |
72.0 |
92.0 - 96.0# |
- |
- |
- |
99.0 |
98.0 |
98.0-99.0# |
CK-MB Subforms
Assay*** |
- |
- |
- |
- |
95.7 |
- |
- |
- |
- |
93.9 - 96.2 |
- |
- |
| #Range was taken from values given for 12, 16, 20, and 24 hours, *[8], ** [9], ***[10] |
Table 2: Sensitivity and specificity of various biomarkers values in %.
CK isoform/subforms
Cytosolic CK exists as a dimer in human tissues composed of two monomers, designated M and B [10,11]. These subunits combine to produce three isoenzymes termed CK-BB, CK-MB, and CK- MM, which are readily distinguishable by electrophoretic mobility [11]. A fourth isoenzyme has been isolated from the mitochondria of mammalian tissues and has been shown to differ from the cytoplasmic forms significantly [11]. CK-BB is predominately localized to brain tissue, CK-MM to skeletal and cardiac muscle tissue, and CK-MB to heart and brain tissue [12]. There are multiple subforms of CK-MM and CK-MB, represented by posttranslational modifications of the carboxyterminus of the M and B monomers [13]. The most commonly assayed CK subforms in the diagnosis of MI are the CK-MB subforms CK-MB1 and CK-MB2, quantified by the ratio MB2/MB1 [10,14].The sensitivity and specificity of CK isoforms increase in early AMI when CK-MB/total CK (relative index) is combined with the results from MB2/MB1 subform analysis [14]. CK-MB remains valuable for the early diagnosis of AMI and holds promise in diagnosing CVA.
Clinical applications
Myocardial infarction: According to the WHO European MI registry criteria, MI can be confidently diagnosed in the presence of one of the following: (1) Electrocardiogram (EKG) showing unequivocal pathological Q waves and/or ST segment elevation >1 mm in contiguous leads with reciprocal changes; (2) history of typical or atypical angina pectoris, together with equivocal changes on the EKG and elevated enzymes; (3) history of typical angina pectoris and elevated enzymes with no changes on the EKG or not available; (4) fatal cases, whether sudden or not, with obvious appearances of fresh MI and/or recent coronary occlusion at autopsy [15].
The release kinetics of these biomarkers provide insight into the time-course of MI and complement a novel, diagnostic algorithm (Figure 1) in patients presenting with MI symptoms. Evidence indicates that a multi-marker strategy, employing a diverse set of biomarkers, adds to risk assessment in acute coronary syndrome (ACS) [16].
Release kinetics of the proposed biomarker dyad are as follows: clinically detectable CK-MB levels begin to rise minutes to hours after symptom onset, peak in 15 and 28 hours in non-Q wave/Q-wave MI, respectively, and fall to < 5% of total CK in 48 hours; clinically detectable Tn serum levels begin to rise in 0-4 hours of necrosis, reach peak levels in 12 to 24 hours, and return to baseline in 4 to 7 days for cTnI and 10 to 14 days for cTnT [12,16].
Reinfarction: Infarction after PCI, fibrinolytic therapy, and coronary artery bypass grafting (CABG) has been difficult to quantify using the current “gold-standard” in the detection of MI. This is a direct result of the biologic compartmentalization of cTn compared to other cardiac biomarkers. Myoglobin and CKMB are localized to the “cytosolic pool” while Tn shares cytosolic expression with a structural, myofibril component [1]. After the initial release of the cytosolic content of the cardiomyocyte and subsequent peaks of the associated biomarkers, the sarcomere begins to break down, releasing the structural reserve of Tn, which explains prolonged Tn elevation [1]. Studies on the release kinetics of myoglobin and CK-MB, state that myoglobin and CK-MB return to normal levels quicker than Tn [17,18]. Therefore, faster, noninvasive confirmation of reinfarction, requiring fewer points of analysis, can be achieved when using CK-MB. CK-MBmass >3-times the norm, post-PCI, and >5-times the norm, post-CABG, is considered a MI [19]. If baseline Tn values are normal, the ESC recommendation is a five-factor increase in Tn post-PCI and ten-factor increase in Tn post-CABG to diagnose MI [20].
Skeletal muscle trauma: Cardiac biomarkers rise in response to skeletal muscle trauma. Prolonged exercise can increase cardiac biomarkers to the level released in minor MI [21,22]. In a 2008 study by Mingels et al., (2009), immediately after a marathon, all runners studied showed an approximate ten-factor increase in cTnT and cTnI concentrations using the hs-cTnT and cTnI assays, respectively [21]. The cTnI assay yielded cTnI concentrations that were increased to greater than the 99th percentile in 81% of the runners [21]. CK-MBmass and myoglobin levels were significantly elevated above pre-race levels in all runners between 1-128 hours post-race; correlation analysis indicated skeletal muscle, not cardiac muscle, as the source of raised serum levels [22].
Over the last 30 years, little has changed in regard to the use of CK isoforms/isoenzymes in certain skeletal muscle diseases. In a study by Wu et al., a patient with scleroderma was evaluated [23]. Compared to normal levels, the patient had elevated CKMB (38.2%) as well as an elevated isoform ratio (2:3) [23]. CKMM and total CK remain important for diagnosing, treating, and following Duchenne muscular dystrophy, polymyositis, dermatomyositis, and rhabdomyolysis [12,24].
Renal insufficiency: Cardiovascular disease accounts for roughly 50% of deaths in patients with chronic renal failure (CRF) [25,26]. In addition, CRF can present asymptomatically, described by: absence of angina and EKG unreliability (resulting from electrolyte disturbances) and conduction abnormalities [25]. The mechanism of elevated Tn is likely due to impaired renal clearance of elevated TnT fragments [27]. However, it is agreed that cTn elevations are associated with increased mortality in patients with CRF [25,26,28]. Subramanian et al. determined total CK elevation, in trauma patients, is correlated with the development of acute renal failure; they reported a sensitivity and specificity for total CK of 70% and 69%, respectively (95% CI; 67-89%) on the first day [29]. Elevated total CK (CK-MM greater than 97% of total CK) has been noted in dialysis patients, but degree of elevation is likely related to muscle mass [30].
Sepsis syndromes: ver Elst et al. observed increased levels of cTnI, cTnT, and CK-MBmass (above the cutoffs in 50%, 36%, and 41% of patients, respectively) in patients presenting with sepsisinduced myocardial dysfunction [31]. In a study by Mehta et al., cTnI, elevated in 43% of patients with septic shock, was, purportedly, a more sensitive biomarker of sepsis-induced myocardial dysfunction than cTnT or CK-MB [31,32]. No prospective study has looked at the use of Tn and/or CK isoenzymes to diagnose sepsis. However, studies have been done on sepsis-suspected patients where biomarkers were drawn to diagnose possible concomitant MI [31-33]. These analyses are discussed later, in the ‘Myocardial Infarction’ section.
Cardiac biomarkers in early diagnosis of CVA: CVA is defined by the WHO as “rapidly developing clinical signs of focal (or global) disturbance of cerebral function, with symptoms lasting 24 hours or longer with no apparent cause other than of vascular origin” [34]. Most of the diagnostic process is based on modern neuroimaging techniques such as computed tomographic (CT) scanning and magnetic resonance imaging (MRI). However, CT scanning has a relatively low sensitivity for acute stroke, 26% (95% CI; 20 to 32%), compared to MRI with a sensitivity of 83% (95% CI; 78 to 88%) [35]. With the early use of thrombolytics today, clinicians require a rapid diagnosis of CVA that differentiates from transient ischemic attack (TIA). Neuroimaging takes significant time in a CVA patient that needs early treatment [36]. Using serum biomarkers may help in reducing the time it takes to diagnose CVA vs. TIA. These potential biomarkers must provide information on whether CVA has occurred and eventually predict the extent of injury.
In a study by Hamburg et al., CK-MM was detected in human brain using polymerase chain reaction amplification of DNA, immunohistochemical evaluation, amino acid sequencing, and gel electrophoresis [37]. The findings showed that approximately 35% of the CK in the hippocampus was CK-MM, although this may have been overestimated, as CK-BB can denature more quickly and is less stable, compared to CK-MM, in certain conditions [37].
Small studies have been done involving CVA patients, describing increased serum and cerebrospinal fluid CK isoenzyme elevation following neurologic insult. For example, Ay et al. conducted a study that included 32 patients with large hemispheric CVA [38]. They reported elevated serum CK-MB, myoglobin and total CK in 11, 26, and 20 of these patients, respectively [38]. Another study done by Kiranmayi et al. looked at 50 patients with acute ischemic CVA. In this study they found 28% of the studied patients had elevated CK-MB; it was hypothesized that CK-MB was of non-cardiac origin in these patients [39]. A third study, by Norris et al., reported, out of 230 CVA patients, 101 had elevated serum levels of total CK, 25 had elevated serum levels of CK-MB, and 0 patients had increased CK-BB levels in serum [40].
Discussion
Myocardial Infarction
Algorithm: Prior to the advent of hs-Tn assays, a single Tn positive blood test could not diagnose a MI secondary to chronic elevations that could be seen in renal failure, tachycardia and certain chronic illnesses. Irreversible cardiac injury (necrosis) can occur within 90 minutes of MI onset, thus early treatment to obtain coronary artery patency is critical to save myocardial muscle [41]. If a patient has classic chest pain coupled with STelevation MI, a Tn measurement is unnecessary and the patient should proceed for emergent percutaneous coronary intervention (PCI) or thrombolysis. If a patient has typical angina and no EKG abnormalities or ST segment depression >1 mm in multiple contiguous leads, then TnT should be ordered at 1 and 3 hours post symptom-start. If Tn is positive on the initial test, CK-MB and total CK should be obtained. If CK-MB is >14 IU/dL and >5% of total CK, then type-I MI is diagnosed. Of note, CK-MB subforms can be assayed at 6 hours post-symptoms. CK-MB subforms >95% sensitivity and within 93 to 96% specificity may be used to diagnose type-I MI [10]. If infarct sizing or reinfarction is of concern, serial testing of CK-MB and total CK should be done, every 6 hours for 24 hours, post-start of symptoms. If hs-TnT is negative at 1 and 3 hours, no further cardiac enzymes should be obtained unless EKG and/or symptoms change. If type-II MI (demand ischemia) is a concern, serial testing of CK-MB should be done as described above to rule in or rule out a concurrent type-I MI. Of course, the cardiac enzymes determinations are only additive to the paramount importance of clinical judgement of the practitioner in all clinical scenarios.
In type-II MI, a 1-hour and 3-hour hs-Tn assay is suggested. If Tn levels elevate or decrease significantly then chronic disease etiologies, renal failure, and tachycardia are less likely. We advocate for serial CK-MB and total CK testing in this scenario or one CK-MB subform measurement 6 hours after symptom onset, as “significant Tn rise or fall” has not been defined well (Figure 1).
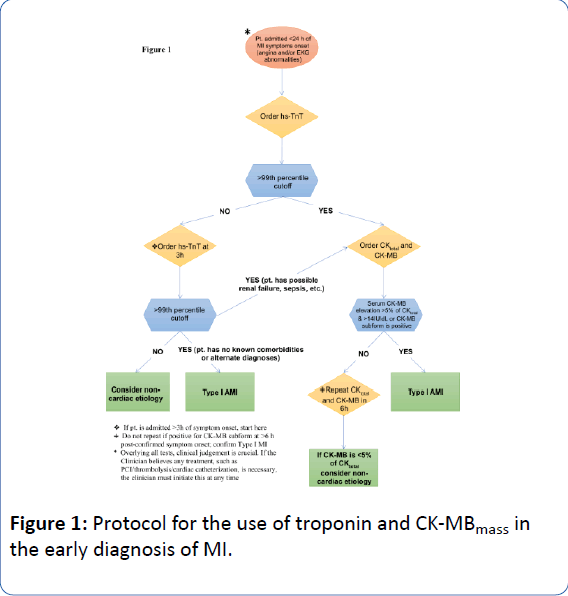
Figure 1: Protocol for the use of troponin and CK-MBmass in the early diagnosis of MI.
Applications of high-sensitive assays and CK-MB: Some nonhigh- sensitivity Tn assays lack antibody specificity, assay precision, and standardization [42]. When using a non-hs-Tn assay, patients seeking care early in the course of their myocardial injury can have undetectable levels of cTn on initial evaluation but will later have a positive cTn result [42]. Given the central clinical goal of rapid screening for patients with chest pain syndromes and the potential benefits of early intervention, this delay in a detectable rise of Tn using conventional assays has led to high clinical interest in using high-sensitivity assays [43].
The advent of hs-Tn assays has supplanted CK-MB as the "gold standard" in chest pain, as evident in the 1-hour and 3-hour protocol suggested by the European Study Group [44]. The specificity of the type of MI, using hs-Tn assays, has caused confusion amongst clinicians and caused unnecessary workups in hospitals, especially in type-II MI (demand ischemia) [45,46]. We suggest the use of serial CK-MB assays for increased specificity in diagnosing MI (type-I vs. type-II), infarct sizing, and reinfarction.
In type-I MI, hs-Tn assays are excellent for initial diagnosis but are not definitive for reinfarction or infarct sizing [7,43]. If patients have subacute thrombosis post-stenting in ST-elevation MI, the clinician needs serial CK-MB assays to help diagnose reinfarction in these patients [47]. Tn levels may remain elevated for weeks, limiting its use in infarct sizing and reinfarction [16,18]. However, CK-MB isoforms have been validated in reinfarction and infarct sizing [9]. Because CK-MB typically returns to normal levels in 48–72 hours after AMI, it aids in the rapid discrimination of reinfarction when symptoms recur between 0 hours and 2 weeks after the initial MI, while Tn may still be increased from the initial cardiac event [16]. Values for measuring infarct size using CK–MB are as follows: Small (CK–MB activity 9 to 60 U/L), Middle (CK–MB activity 61 to 120 U/L), and Large (CK-MB activity >120 U/L) [9]. Both reinfarction and infarct sizing are important to clinicians in terms of prognosis and treatment.
Type-II MI (demand ischemia) has been a confounder for clinicians. In some clinical scenarios, Tn elevation in marathon runners, stress testing, and renal failure may be misclassified as MI [25,48]. We know these clinical conditions may have negative CK-MB (percent of total), negative stress myocardial perfusion imaging and/or no change in left ventricular function. It is likely that Tn elevation in these situations is not due to significant infarction [21,25,48]. We suggest serial evaluations of CK-MB isoforms/subforms and total CK at 6 to 8 hours after symptom onset to define the type of MI and begin to measure infarct size. Mortality has been shown to increase in MI when CK-MB is >5% of total CK and >14 IU/dL [12]. The use of serial CK-MB isoforms/ subforms to diagnose non-cardiac conditions will save patient workups and morbidity from unnecessary evaluations and expense.
According to the diagnostic criteria for AMI, a hs-cTnT has a NPV of 99% (95% CI; 97% to 100%) (Table 1) [7]. The NPV of hs- Tn in clinical scenarios is of value and may be useful in prognosis. By using this value, patients are less likely to be inappropriately diagnosed with ACS, unstable angina, or clinically significant coronary artery disease. Since TnT is elevated in many ACS, tachycardia, pulmonary emboli, and other patients, using the NPV for cTnT may lead to shorter hospital stays and workups with a better prognosis [49-51]. If Tn is negative on serial measurements, CK-MB does not need to be tested, the prognosis is much improved, and coexistent MI is ruled out [15,52].
When necessary, performing serial CK-MB assays will limit differential diagnosis amongst clinicians and delineate clinically important infarct size and reinfarction. It is evident that CK-MB isoform measurements are important to rule out ACS in patients suspected of skeletal muscle trauma and/or CRF, as a result of cTn elevated to levels above normal. The economics of focusing on the correct diagnosis, rapid discharge, and extent of disease will significantly reduce healthcare dollars and potentially decrease patient morbidity.
CVA: CK-MM is phenotypically expressed in human brain and it is known that CK-BB is present in human brain as well [12]. Significant quantities of non-sarcomeric mitochondrial CK have also been detected [12]. Therefore, it is likely that CK isoenzymes are released in acute CVA [12,38,53]. Studies cited suggest CK isoenzymes are elevated in many post-CVA patients, although some researchers suggest concomitant MI may be the cause [37-40,54]. The sensitivity of CK isoenzyme elevation is variable and likely assay dependent. Elevated Tn in CVA, likely due to concurrent MI, may be of value in that it suggests worse prognosis [55]. Tn assays in CVA have not been validated as being released from the brain. Specificity of Tn elevation in CVA limits the advocacy for serial Tn assays in acute CVA [38]. We suggest more research is needed to establish the more plausible etiology that CK release is CVA-specific, and that serial CK isoforms (CK-MMmass, CK-MB, CK-BBmass, and percent of total CK), post-CVA may improve rapid diagnosis. This mirrors early studies of CK and CK-MB in AMI before these were defined. Due to the lack of large studies and the mostly descriptive nature of the literature on CK isoenzyme release into serum following CVA, sensitivity and specificity cannot be reported definitively. The percent of CK-MB, CK-MM, and CK-BB has yet to be defined in acute CVA. We suggest that these values need to be defined to the same degree as in AMI. Doing so could lead to the rapid diagnosis of CVA and rule out TIA, by CK-isoform relative index. This would direct thrombolytic treatment to the correct patients and limit morbidity/mortality in TIA patients.
Using troponin to predict prognosis: Any Tn elevation in MI, renal failure, sepsis, and CVA has a worse prognosis compared to no Tn elevation [55,56]. Roos et al. conducted an observational study using a cohort of 19,460 patients looking at all-cause mortality in relation to Tn levels, with a mean follow-up of 3.3 ± 1.2 years [56]. A hs-cTnT assay was used and patients were categorized into 6 groups based on their measured TnT levels: <5 ng/L (reference group), 5-9 ng/L, 10-14 ng/L, 15-29 ng/L, 30-49 ng/L, ≥ 50 ng/L [56]. Compared to the reference group, the adjusted risk of death for the 5-9 ng/L group increased approximately 2-fold, increased 3-fold for the 10-14 ng/L group, and increased by almost 10-fold for the ≥ 50 ng/L group; the adjusted risk for cardiovascular mortality increased 27-fold for patients with TnT levels ≥ 50 ng/L, compared to patients with <5 ng/L [56]. This increased morbidity is likely due to comorbid conditions of demand ischemia or AMI [56]. When treating a patient with these conditions, we suggest drawing at least one Tn assay during the patient's hospital stay for prognostic consideration and possible comorbid myocardial disease.
Conclusion
Currently, hs-TnT is the “gold standard” for diagnosing type-I AMI within 1-3 hours post-symptom onset. Using multiple cardiac biomarkers can help measure infarct size, help determine reinfarction, and improve sensitivity and specificity in the diagnosis of MI. Our contemporary algorithm can improve diagnostic accuracy for MI and MI-type. The NPV of Tn is useful in determining patient prognosis. CK-MB isoforms are superior to Tn in infarct sizing and should be used post-PCI/post-CABG to evaluate reinfarction. Future studies with CK isoforms may lead to rapid diagnosis of CVA vs. TIA. This could aid thrombolytic decisions and decrease morbidity and mortality.
Competing Interests
All authors do not have any competing interests
22307
References
- Babuin L, Jaffe A (2005) Troponin: the biomarker of choice for the detection of cardiac injury. CMAJ 173: 1191-1202.
- Apple F, Wu A, Jaffe A (2002) European Society of Cardiology and American College of Cardiology guidelines for redefinition of myocardial infarction: How to use existing assays clinically and for clinical trials. Am Heart J 144: 981-986.
- Ferguson J, Beckett G, Stoddart M, Walker S, Fox K (2002) Myocardial infarction redefined: The new ACC/ESC definition, based on cardiac troponin, increases the apparent incidence of infarction. Heart 88: 343-347.
- Panteghini M, Pagani F, Yeo K, Apple F, Christenson R, et al. (2004) Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem 50: 327-332.
- Herman D, Kavsak P, Greene D (2017) Variability and error in cardiac troponin testing: An ACLPS critical review. Am J Clin Pathol 148: 281-295.
- Christenson R, Jacobs E, Uettwiller-Geiger D, Estey M, Lewandrowski K, et al. (2017) Comparison of 13 commercially available cardiac troponin assays in a multicenter north american study. J Appl Lab Med 1: 544-561.
- Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, et al. (2009) Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med 361: 858-867.
- Tucker J, Collins R, Anderson A, Hauser J, Kalas J, et al. (1997) Early diagnostic efficiency of cardiac troponin I and troponin T for acute myocardial infarction. Acad Emerg Med 4: 13-21.
- de Winter R, Koster R, Sturk A, Sanders G (1995) Value of myoglobin, troponin T, and CK-MBmass in ruling out an acute myocardial infarction in the emergency room. Circulation 92: 3401-3407.
- Puleo P, Meyer D, Wathen C, Tawa C, Wheeler S, et al. (1994) Use of rapid assay of subforms of creatine kinase MB to diagnose or rule out acute myocardial infarction. N Engl J Med 331: 561-566.
- Panteghini M (1988) Serum isoforms of creatine kinase isoenzymes. Clin Biochem 21: 211-218.
- Hamburg R, Friedman D, Perryman M (1991) Metabolic and diagnostic significance of creatine kinase isoenzymes. Trends Cardiovasc Med 1: 195-200.
- Adams J, Abendschein D, Jaffe A (1993) Biochemical markers of myocardial injury. Is MB creatine kinase the choice for the 1990s?. Circulation 88: 750-763.
- Wu A (1992) Creatine kinase MM and MB isoforms. Lab Medicine 23: 303-305.
- Mendis S, Thygesen K, Kuulasmaa K, Giampaoli S, Mähönen M, et al. (2011) World health organization definition of myocardial infarction: 2008-09 revision. Int J Epidemiol 40: 139-146.
- Morrow D, Cannon C, Jesse R, Newby L, Ravkilde J, et al. (2007) National academy of clinical biochemistry laboratory medicine practice guidelines: Clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation 115: 356-375.
- Kehl D, Igbal N, Fard A, Kipper B, De La Parra Landa A, et al. (2012) Biomarkers in acute myocardial injury. Transl Res 159: 252-264.
- Katus H, Remppis A, Scheffold T, Diederich K, Kuebler W (1991) Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol 67: 1360-1367.
- French J, White H (2004) Clinical implications of the new definition of myocardial infarction. Heart 90: 99-106.
- Thygesen K, Alpert J, Jaffe A, Simoons M, Chaitman B, et al. (2012) Third universal definition of myocardial infarction. Circulation 126: 2020-2035.
- Mingels A, Jacobs L, Michielsen E, Swaanenburg J, Wodzig W, et al. (2009) Reference population and marathon runner sera assessed by highly sensitive cardiac troponin T and commercial cardiac troponin T and I assays. Clin Chem 55: 101-108.
- Cummins P, Young A, Auckland M, Michie C, Stone P, et al. (1987) Comparison of serum cardiac specific troponin-I with creatine kinase, creatine kinase-MB isoenzyme, tropomyosin, myoglobin and C-reactive protein release in marathon runners: cardiac or skeletal muscle trauma?. Eur J Clin Invest 17: 317-324.
- Wu A, Wang X, Gornet T, Ordóñez-Llanos J (1992) Creatine kinase MB isoforms in patients with skeletal muscle injury: ramifications for early detection of acute myocardial infarction. Clin Chem 38: 2396-2400.
- Chemnitz G, Schmidt E, Schmidt F (1981) Collagen diseases. Creatine kinase isoenzymes: pathophysiology and clinical applications. Berlin: 190-194.
- Freda B, Tang W, Van Lente F, Peacock W, Francis G (2002) Cardiac troponins in renal insufficiency: Review and clinical implications. J Am Coll Cardiol 40: 2065-2071.
- Apple F, Murakami M, Pearce L, Herzog A (2002) Predictive value of cardiac troponin I and T for subsequent death in end-stage renal disease. Circulation 106: 2941-2945.
- Diris J, Hackeng C, Kooman J, Pinto Y, Hermens W, et. al. (2004) Impaired renal clearance explains elevated troponin T fragments in hemodialysis patients. Circulation 109: 23-25.
- Khan N, Hemmelgarn B, Tonelli M, Thompson C, Levin A (2005) Prognostic value of troponin T and I among asymptomatic patients with end-stage renal disease: A meta-analysis. Circulation 112: 3088-3096.
- Subramanian A, Sukheeja D, Trikha V, Pandey A, Albert V, et al. (2013) Evaluation of serum creatine kinase and urinary myoglobin as markers in detecting development of acute renal failure in severely injured trauma patients. ISRN Emergency Medicine: 241036.
- Singhal P, Barth R, Ginsberg N, Lynn R (1988) Determinants of serum creatine kinase activity in dialysis patients. Am J Nephrol 8: 220-224.
- ver Elst K, Spapen H, Nguyen D, Garbar C, Huyghens L, et al. (2000) Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 46: 650-657.
- Mehta N, Khan I, Gupta V, Jani K, Gowda R, et al. (2004) Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 95: 13-17.
- McLean A, Huang S (2012) Cardiac biomarkers in the intensive care unit. Ann Intensive care 2: 8.
- Andrade S, Harrold L, Tija J, Cutrona S, Saczynski J, et al. (2012) A systematic review of validated methods for identifying cerebrovascular accident or transient ischemic attack using administrative data. Pharmacoepidemiol Drug Saf 21: 100-128.
- Chalela J, Kidwell C, Nentwich L, Luby M, Butman, J, et al. (2007) Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: A prospective comparison. Lancet 369: 293-298.
- Wardlaw J, Keir S, Dennis M (2003) The impact of delays in computed tomography of the brain on the accuracy of diagnosis and subsequent management in patients with minor stroke. J Neurol Neurosurg Psychiatry 74: 77-81.
- Hamburg R, Friedman D, Olson E, Ma T, Cortez M, et al. (1990) Muscle creatine kinase isoenzyme expression in adult human brain. J Biol Chem 265: 6403-6409.
- Ay H, Arsava E, Sariba? O (2002) Creatine kinase-MB elevation after stroke is not cardiac in origin: Comparison with troponin T levels. Stroke 33: 286-289.
- Kiranmayi B, Bhavani V, Tagore R (2015) Evaluation of CK MB levels in acute ischemic stroke. IOSR-JPBS 10: 38-42.
- Norris J, Hachinski V, Myers M, Callow J, Wong T, et al. (1979) Serum cardiac enzymes in stroke. Stroke 10: 548-553.
- Christenson R, Azzazy H (2006) Biomarkers of myocardial necrosis: Past, present, and future. Cardiovascular biomarkers: pathophysiology and disease management. Totowa: 3-25.
- Melanson S, Morrow D, Jarolim P (2007) Earlier detection of myocardial injury in a preliminary evaluation using a new troponin I assay with improved sensitivity. Am J Clin Pathol 128: 282-286.
- Antman E, Bassand J, Klein W, Ohman M, Sendon J, et al. (2000) Myocardial infarction redefined- a consensus document of the joint european society of cardiology/american college of cardiology committee for the redefinition of myocardial infarction. J Am Coll Cardiol 36: 959-969.
- Pickering J, Greenslade J, Cullen L, Flaws D, Parsonage W, et al. (2016) Validation of presentation and 3 h high-sensitivity troponin to rule-in and rule-out acute myocardial infarction. Heart 102: 1270-1278.
- Collinson P, Lindahl B (2015) Type 2 myocardial infarction: The chimaera of cardiology?. Heart 101: 1697-1703.
- Wildi K, Gimenez M, Twerebold R, Reichlin T, Jaeger C, et al. (2015) Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation 131: 2032-2040.
- Lim C, van Gaal W, Testa L, Cuculi F, Arnold J, et al. (2011) With the “universal definition,” measurement of creatine kinase-myocardial band rather than troponin allows more accurate diagnosis of periprocedural necrosis and infection after coronary intervention. J Am Coll Cardiol 57: 653-661.
- Hubble M, Fatovich D, Grasko J, Vasikaran S (2009) Cardiac troponin increases among marathon runners in the perth marathon: The troponin in marathons (TRIM) study. Med J Aust 190: 91-93.
- Hamm C, Ravkilde J, Gerhardt W, Jorgensen P, Peheim E, et al. (1992) The prognostic value of serum troponin T in unstable angina. N Engl J Med 327: 146-150.
- Brush J, Kaul S, Krumholz H (2016) Troponin testing for clinicians. J Am Coll Cardiol 68: 2365-2375.
- Tanindi A, Cemri M (2011) Troponin elevation in conditions other than acute coronary syndromes. Vasc Health Risk Manag 7: 597-603.
- Ottani F, Galvani M, Nicolini F, Ferrini D, Pozzati A, et al. (2000) Elevated cardiac troponin levels predict the risk of adverse outcome in patients with acute coronary syndromes. Am Heart J 140: 917-927.
- Ingebrigtsen T, Romner B (2002) Biochemical serum markers of traumatic brain injury. J Trauma 52: 798-808.
- Mooe T, Erikkson P, Stegmayr B (1997) Ischemic stroke after acute myocardial infarction. A population-based study. Stroke 28: 762-767.
- Raza F, Alkhouli M, Sandhu P, Bhatt R, Bove A (2014) Elevated cardiac troponin in acute stroke without acute coronary syndrome predicts long-term adverse cardiovascular outcomes. Stroke Res Treat: 621650.
- Roos A, Bandstein N, Lundback M, Hammarsten O, Ljung R, et al. (2017) Stable high-sensitivity cardiac troponin T levels and outcomes in patients with chest pain. J Am Coll Cardiol 70: 2226-2236.







