Research Article - (2023) Volume 15, Issue 3
Cortical gray matter structural changes in obese on 3T MRI
Hyeon-Man Baek*
Department of Health Sciences and Technology, Gachon University, Incheon, Korea
*Correspondence:
Hyeon-Man Baek, Department of Health Sciences and Technology, Gachon University, Incheon,
Korea,
Tel: +82-010-9878-4279,
Email:
Received: 24-Apr-2023, Manuscript No. ipaom-23-13726;
Editor assigned: 26-Apr-2023, Pre QC No. P-13726;
Reviewed: 08-May-2023, QC No. Q-13726;
Revised: 13-May-2023, Manuscript No. R-13726;
Published:
20-May-2023
Summary
Background: Obesity is associated with imbalanced energy intake
and expenditure. Many studies have been conducted on obesity
and brain morphometric structural changes. However, the findings
have been inconsistent and the exact relationships between obesity
and brain structural changes are unclear. We aimed to examine the
structural alterations of cortical gray matter between obese patients
and healthy controls with normal weight. Methods: 21 obese
patients (age=24.05 ± 3.41 years; body mass index [BMI]=29.81 ±
3.89 kg/m2) were age-matched with 17 healthy controls (age=25.65
± 4.29 years; [BMI]=22.46 ± 1.43 kg/m2). High-resolution T1-weighted
MPRAGE 3D scans were acquired on a 3T MRI scanner. FreeSurfer
and FSL-FIRST were used to examine cortical thickness, surface area,
and volume. Results: The obese patients exhibited increased and
decreased cortical structural brain alterations in frontal, parietal,
temporal, and occipital cortex area summarize the article’s main
findings. Our results suggest that morphological changes of brain
structures can lead to functional variations that worsen food intake
behavior. Conclusions: The current study presents the association
between obesity and structural brain differences in several cortical
gray matter regions involved in food intake regulation. Therefore,
we believe that alterations in brain structure could be neuronal
markers in understanding how obesity develops.
Introduction
Obesity is one of the major public health issues with
rates nearly tripling over the past three decades. In 2016,
39% of adults aged 18 years and over were overweight and
13% were obese [1]. The increasing emergence of obesity
is associated with multiple morbidities, including an type
2 diabetes [2], hypertension [3], cardiovascular disease [4],
and cancer [5].
When energy intake exceeds a person’s energy
expenditure, excess energy contributes to weight gain
[6]. Obesity results from changes in homeostasis and
sybaritic food intake behavior resulting from changes in
the plasticity of cortical and subcortical brain structures
[7]. Therefore, unnatural eating habits are an important
factor in defining obesity as a disease [8]. Food intake
is modulated by various cognitive influences such as
celebratory representation, environmental situations, and
emotional and compensatory characteristics [9]. Studies
have shown that structural differences in the brain may
cause a more likelihood of obesity, but it is also likely that
the condition of obesity itself can change the brain due to
development of physiological control disorders [10].
A number of studies have reported that increased
body mass index (BMI) is related to the increased
cortical thickness and that there was a significant positive
correlation between visceral fat ratio and cortical thickness
throughout the brain [11,12]. In contrast, other studies
showed that increasing BMI was associated with cortical
thinning in the left inferior temporal and the inferior
parietal cortex and increasing visceral adipose tissue was
related to cortical thinning in the left fusiform gyrus, the
right inferior temporal and mid-insular [13]. BMI showed
a negative correlation with cortical thickness in the left
lateral occipital cortex and right ventromedial prefrontal
cortex area [10].
Studies that assessed gray matter volume (GMV) in
obese subjects found that obese subjects showed enlarged
left putamen which correlated with increasing BMI and
enlarged amygdala and hippocampus [8,14]. Studies
also showed that the higher waist-hip ratio and waist
circumference, the lower the total brain volume (TBV) and
GMV [15]. Previous studies that compared lean subjects
and obese individuals showed that obese individuals had
significant lower gray matter density in the postcentral
gyrus, frontal operculum, putamen, middle frontal gyrus
[16], left dorsolateral prefrontal cortex [17], ventral
diencephalon, and brainstem than those of lean subjects [7].
A number of studies have suggested the relationships
between obesity and morphology of the brain area.
However, the results of previous research are diverse and
consistent results on regional brain changes in obesity have
not been established. The purpose of this study was to
examine the structural differences of the cortical gray matter
(e.g., cortical thickness, surface area, and volume) between
obese patients and healthy controls. We hypothesized that
obese patients would exhibit regional cortical structural
alterations in brain areas which are involved in food intake
behavior regulation.
Materials and Methods
Subjects
Thirty-eight male subjects, 21 with obese (age=24.05 ±
3.41 years; body mass index [BMI]=29.81 ± 3.89 kg/m2)
and 17 healthy controls with normal weight (age=25.65
± 4.29 years; [BMI]=22.46 ± 1.43 kg/m2) were recruited
from Chungbuk National University. BMI was calculated
as body weight in kilograms divided by the square of
height in meters. Obesity was designated as a BMI ≥
25.0 kg/m2 using the adjusted Korean guideline. Subjects
with neurological abnormalities, history of psychiatric
illnesses, illicit drug dependence or alcohol abuse were
excluded from this study. This study was approved by the
Institutional Review Board (IRB) by College of Medicine
Chungbuk National University in Cheongju, Korea. All
subjects provided written informed consent after detailed
instructions of the study.
MRI acquisition
Brain imaging data were acquired on a 3T MR scanner
(Achieva 3.0T TX, Philips Medical Systems, Eindhoven,
Netherlands). A 32-channel receive-only phased array
head coil was used for receiving. Structural images were
acquired using a high-resolution T1-weighted threedimensional
(3D) magnetization prepared rapid gradient
echo (MPRAGE) with the following parameters: repetition
time (TR)=7 ms, echo time (TE)=3 ms, flip angle=9º, slice
thickness=1.2 mm, field of view (FOV)=256 mmⅹ 256
mm, and matrix=243.
Image processing
T1-weighted MR images were converted from
DICOM to NIFTI files using MRIcron software (http://
www.cabiatl.com/micro/mricron/index.html, accessed on
19 August 2008) [18] and processed using FreeSurfer image
analysis suite (v6.0.0, http://surfer.nmr.mgh.harvard.
edu/, accessed on 1 January 2017). The entire process is
completely automated. The detailed pipeline for FreeSurfer
is described on the web page. Briefly, T1-weighted MR
images were linearly registered to the Talairach space,
B1 bias field corrected and skull stripped. Images were
segmented into the gray-white matter and reconstructions
of cortical surface models were identified using gray-white
boundary surface and pial surface. The regions on the
cortical surface, as well as subcortical brain structures, were
labeled by nonlinear registration of the cortical surface of
a subject with a stereotaxic atlas. FreeSurfer output was
visually checked to ensure accuracy and image quality.
Statistical analysis
Group differences in age, BMI, GMV, white matter
volume, TBV were analyzed with one-way ANOVA using
IBM® SPSS® Statistics (version 23). The statistical threshold
was set at p<0.05.
Group cortical analysis was performed to compare
cortical thickness, cortical surface area, and cortical volume
between groups. Statistical maps were generated using
general linear models (GLM) in FreeSurfer’s statistical tool
QDEC (Query, Design, Estimate, Contrast), including age
as a nuisance factor. The significant threshold was set at
voxel-wise p<0.01 and cluster-wise p<0.05, Monte Carlo
correction with 10,000 iterations for multiple comparisons.
Data were smoothed with a Gaussian 10 mm full-width-athalf-
maximum (FWHM) kernel to enhance the variability
between subjects [19].
Results
Demographic and clinical characteristics are presented
in Tab.1. The obese patients and healthy controls differed
significantly in terms of BMI (obesity patients=29.81 ±
3.89; healthy controls=22.46 ± 1.43; p<0.001). There were
no significant group differences in age (p=0.208), GMV
(p=0.177), white matter volume (p=0.848) and total brain
volume (p=0.356).
| Characteristic |
Obese patients
(N=21) |
Healthy controls
(N=17) |
p-value |
| Age (year) |
24.05 (3.41) |
25.65 (4.29) |
0.208 |
| Body Mass Index (kg/m2) |
29.81 (3.89) |
22.46 (1.43) |
<0.001* |
| Gray Matter volume (cm3) |
825.09 (38.12) |
809.40 (30.55) |
0.177 |
| White Matter volume (cm3) |
702.38 (31.37) |
700.21 (37.99) |
0.848 |
| Total Brain volume (cm3) |
1527.47 (55.58) |
1509.61 (62.10) |
0.356 |
Tab. 1. Demographic and clinical characteristics between obese patients and healthy controls.
Examples of cortical thickness differences between
obese patients and healthy controls, after controlling age,
are shown in Fig. 1. Thirty-five clusters of thinner regions
and eight clusters of thicker regions were observed in obese
patients (p<0.01). Representations of these regions are
shown in Tab. 2.
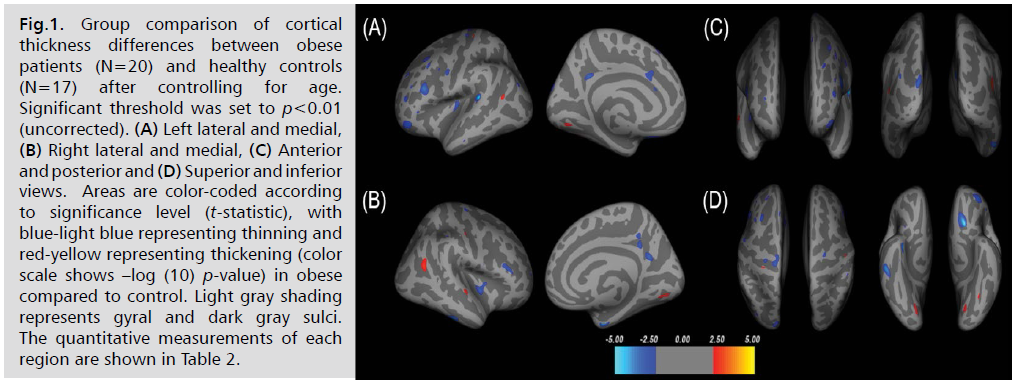
Fig 1:Group comparison of cortical thickness differences between obese patients (N=20) and healthy controls (N=17) after controlling for age. Significant threshold was set to p<0.01 (uncorrected). (A) Left lateral and medial, (B) Right lateral and medial, (C) Anterior and posterior and (D) Superior and inferior views. Areas are color-coded according to significance level (t-statistic), with blue-light blue representing thinning and red-yellow representing thickening (color scale shows –log (10) p-value) in obese compared to control. Light gray shading represents gyral and dark gray sulci. The quantitative measurements of each region are shown in Table 2.
| |
Max t-statistic |
Size (mm2) |
Talairach Coordinates |
N vertices |
Anatomical Regions |
| X |
Y |
Z |
| Obese>Control |
2.7141 |
113.23 |
41.9 |
-57.7 |
16.7 |
249 |
R inferior parietal |
| 2.6828 |
34.72 |
-44.5 |
-61 |
11.9 |
71 |
L inferior parietal |
| 2.4766 |
35.78 |
63.5 |
-10.7 |
-1.7 |
90 |
R superior temporal |
| 2.3338 |
93.39 |
-16.5 |
-73.6 |
-9.7 |
106 |
L lingual |
| 2.2591 |
17.76 |
47.9 |
-18 |
41.6 |
37 |
R postcentral |
| 2.1979 |
121.44 |
18.2 |
-72.3 |
-6.4 |
125 |
R lingual |
| 2.1726 |
20.18 |
-27.9 |
-30.5 |
60.1 |
42 |
L postcentral |
| 2.1217 |
17.15 |
-32.5 |
-61.1 |
-15.5 |
24 |
L fusiform |
| Obese<Control |
-5.2037 |
114.06 |
-53.3 |
-36.9 |
10.3 |
248 |
L superior temporal |
| -5.0148 |
185.28 |
-15.6 |
20 |
-16.9 |
440 |
L lateral orbitofrontal |
| -3.9973 |
58.85 |
25.6 |
-2.9 |
-34.5 |
146 |
R entorhinal |
| -3.9391 |
186.87 |
56.2 |
-30.4 |
-26.9 |
299 |
R inferior temporal |
| -3.6392 |
197.32 |
-39.5 |
22.8 |
20.2 |
364 |
L rostral middle frontal |
| -3.3098 |
201.81 |
-11.7 |
-94.8 |
20.4 |
259 |
L lateral occipital |
| -3.215 |
151.6 |
35.4 |
-12.5 |
-5.2 |
369 |
R insula |
| -3.2074 |
63.77 |
-9.7 |
-49 |
29.3 |
141 |
L isthmus cingulate |
| -3.1925 |
223.97 |
-35.4 |
44.3 |
-10.6 |
322 |
L pars orbitalis |
| -3.1829 |
88.37 |
4.6 |
-58.7 |
21 |
183 |
R precuneus |
| -2.9156 |
156.77 |
-13.2 |
-27.6 |
65.5 |
363 |
L precentral |
| -2.882 |
47.14 |
12.2 |
-44.6 |
31.4 |
129 |
R isthmus cingulate |
| -2.7613 |
44.78 |
-23.8 |
-23.5 |
57.8 |
99 |
L precentral |
| -2.7126 |
70.44 |
-6 |
19 |
28.6 |
157 |
L caudal anterior cingulate |
| -2.7032 |
40.29 |
-55.3 |
-0.5 |
35.6 |
105 |
L precentral |
| -2.6532 |
127.39 |
44.4 |
29.7 |
12.4 |
217 |
R rostral middle frontal |
| -2.5902 |
98.69 |
-33.3 |
-20.5 |
5.6 |
211 |
L insula |
| -2.5663 |
74.59 |
-31.3 |
18.6 |
44.1 |
120 |
L caudal middle frontal |
| -2.434 |
42.56 |
29.6 |
-17.9 |
68.6 |
99 |
R precentral |
| -2.3584 |
17.63 |
-44.7 |
30.8 |
-1.7 |
31 |
L pars triangularis |
| -2.3494 |
48.96 |
-37.9 |
46.1 |
12 |
70 |
L rostral middle frontal |
| -2.3286 |
38.31 |
-39.3 |
21.5 |
40 |
67 |
L caudal middle frontal |
| -2.3101 |
41.04 |
6.8 |
-44.5 |
40.6 |
104 |
R precuneus |
| -2.2898 |
29.47 |
-38.8 |
-81.5 |
24.6 |
46 |
L inferior parietal |
| -2.2448 |
4.61 |
-5.8 |
25.1 |
17.2 |
12 |
L caudal anterior cingulate |
| -2.2244 |
42 |
-24 |
39.4 |
32.3 |
63 |
L rostral middle frontal |
| -2.1218 |
14.09 |
25.1 |
52.7 |
8.9 |
19 |
R rostral middle frontal |
| -2.1206 |
12.69 |
49.2 |
-36.2 |
45.8 |
26 |
R supramarginal |
| -2.1086 |
17.33 |
-7 |
28.8 |
54.8 |
32 |
L superior frontal |
| -2.0969 |
12.27 |
-40.9 |
38.8 |
23.1 |
19 |
L rostral middle frontal |
| -2.088 |
4.1 |
28.5 |
12.3 |
-15.7 |
13 |
R insula |
| -2.0466 |
6.19 |
-61.1 |
-17.4 |
-22.7 |
8 |
L middle temporal |
| -2.039 |
4.51 |
-36.6 |
1.1 |
33.5 |
10 |
L caudal middle frontal |
| -2.025 |
1.9 |
55.8 |
4.9 |
14.2 |
4 |
R precentral |
| -2.0164 |
2.23 |
37.9 |
-16 |
-28.8 |
4 |
R fusiform |
*Positive t-values indicate thickening and negative values indicate thinning. X/Y/Z represent Talairach Coordinates in millimeters. N vertices are vertex number at maximum. Results are shown with a threshold of p<0.01. Group comparisons including age as nuisance factor. L, left; R, right.
Tab. 2. Clusters of significant differences in cortical thickness in obese patients compared to healthy controls.
Compared with the healthy controls controlling for age,
the obese patients showed significant larger cortical surface
area (p<0.01) in the left superior temporal, postcentral,
isthmus cingulate, and precuneus. Also, obese patients
showed the reduced cortical surface area in the left pars
opercularis and right temporal pole (see Fig. 2. and Tab. 3).
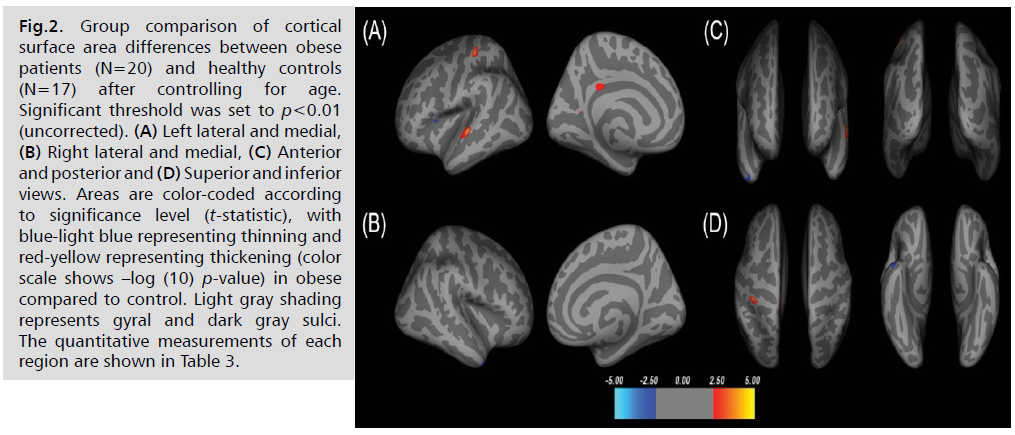
Fig 2: Group comparison of cortical surface area differences between obese patients (N=20) and healthy controls (N=17) after controlling for age. Significant threshold was set to p<0.01 (uncorrected). (A) Left lateral and medial, (B) Right lateral and medial, (C) Anterior and posterior and (D) Superior and inferior views. Areas are color-coded according to significance level (t-statistic), with blue-light blue representing thinning and red-yellow representing thickening (color scale shows –log (10) p-value) in obese compared to control. Light gray shading represents gyral and dark gray sulci. The quantitative measurements of each region are shown in Table 3.
| |
Max t-statistic |
Size (mm2) |
X |
Y |
Z |
N vertices |
Anatomical Regions |
| Obese>Control |
4.0068 |
192.82 |
-62.9 |
-17.8 |
-0.9 |
520 |
L superior temporal |
| 3.7039 |
155.73 |
-43.1 |
-29.5 |
61.8 |
395 |
L postcentral |
| 2.4997 |
69.86 |
-6.7 |
-35.5 |
28.9 |
171 |
L isthmus cingulate |
| 2.036 |
2.84 |
-14.5 |
-57.1 |
10.2 |
8 |
L precuneus |
| Obese<Control |
-2.2619 |
13.8 |
-32 |
15.7 |
13.3 |
35 |
L pars opercularis |
| -2.2145 |
96.29 |
41.5 |
12.6 |
-37.2 |
140 |
R temporal pole |
*Positive t-values indicate thickening and negative values indicate thinning. X/Y/Z represent Talairach Coordinates in millimeters. N vertices are vertex number at maximum. Results are shown with a threshold of p<0.01. Group comparisons including age as nuisance factor. L, left; R, right.
Tab. 3. Demographic and clinical characteristics between obese patients and healthy controls.
After controlling age, there were cortical volume
increases in obese patients in the left postcentral, superior
temporal, postcentral, medial orbitofrontal, isthmus
cingulate, bankssts and right paracentral, lingual, and
bankssts. Cortical volume reductions were observed in the
left pars opercularis, rostral middle frontal, lateral occipital
and right postcentral, superior parietal, rostral middle
frontal, middle temporal, inferior temporal, and lateral
occipital in obese patients compared to healthy controls
with significant threshold of (p<0.01, see Fig. 3. and Tab. 4.).
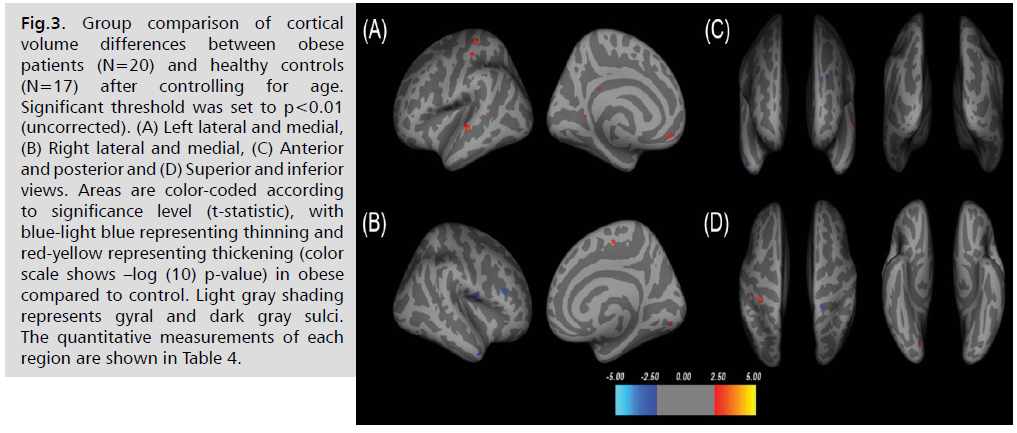
Fig 3: Group comparison of cortical volume differences between obese patients (N=20) and healthy controls (N=17) after controlling for age. Significant threshold was set to p<0.01 (uncorrected). (A) Left lateral and medial, (B) Right lateral and medial, (C) Anterior and posterior and (D) Superior and inferior views. Areas are color-coded according to significance level (t-statistic), with blue-light blue representing thinning and red-yellow representing thickening (color scale shows –log (10) p-value) in obese compared to control. Light gray shading represents gyral and dark gray sulci. The quantitative measurements of each region are shown in Table 4.
| |
Max t-statistic |
Size (mm2) |
X |
Y |
Z |
N vertices |
Anatomical Regions |
| Obese>Control |
3.4062 |
78.18 |
-38.4 |
-31.1 |
65.4 |
236 |
L postcentral |
| 3.2085 |
127.01 |
-58.9 |
-20.5 |
-2.3 |
289 |
L superior temporal |
| 2.8765 |
47.07 |
-48.4 |
-25.3 |
55.8 |
112 |
L postcentral |
| 2.4903 |
29.6 |
-11.3 |
40.2 |
-11.2 |
59 |
L medial orbitofrontal |
| 2.2869 |
18.9 |
7.6 |
-16.9 |
50.2 |
37 |
R paracentral |
| 2.2072 |
49.47 |
11.9 |
-82.5 |
-9.6 |
38 |
R lingual |
| 2.0745 |
6.91 |
-15.2 |
-51.5 |
4.6 |
17 |
L isthmus cingulate |
| 2.062 |
6.54 |
44.9 |
-41.3 |
2.9 |
23 |
R bankssts |
| 2.0511 |
3.53 |
-9 |
-38.6 |
26.5 |
19 |
L isthmus cingulate |
| 2.0411 |
4.8 |
-53.5 |
-42.1 |
6.1 |
11 |
L bankssts |
| Obese<Control |
-3.0252 |
104.62 |
52.4 |
-7 |
11.1 |
251 |
R postcentral |
| -2.8471 |
52.21 |
16.9 |
-39.1 |
66.7 |
102 |
R superior parietal |
| -2.7637 |
47.93 |
43.5 |
26 |
19.2 |
90 |
R rostral middle frontal |
| -2.2506 |
28.75 |
51.4 |
5.8 |
-30.8 |
44 |
R middle temporal |
| -2.2094 |
10.63 |
-33.6 |
23.2 |
11.1 |
32 |
L pars opercularis |
| -2.178 |
14.46 |
50.2 |
-33.4 |
-24.3 |
22 |
R inferior temporal |
| |
-2.1588 |
27.62 |
-24 |
41.2 |
29.5 |
36 |
L rostral middle frontal |
| -2.0874 |
11.82 |
46.1 |
-65.9 |
-11.3 |
14 |
R lateral occipital |
| -2.0598 |
7.77 |
-31.9 |
-81.5 |
-12.8 |
10 |
L lateral occipital |
Tab. 4. Clusters of significant differences in cortical volume in obese patients compared to healthy controls.
Discussion
In the present study, structural brain alterations in the
cortical gray matter between obese patients and healthy
controls with normal weight were studied. Using FreeSurfer, a surface-based cortical brain measurement application,
we analyzed cortical thickness, cortical surface area, and
cortical volume between groups. We found that several
cortical regions including frontal, parietal, temporal, and
occipital cortex were altered in obese patients. These results
show evidence of the structural brain alterations that occur
within obese subjects.
Our results show that obese patients exhibit cortical
thickening in the parietal, temporal, and occipital cortex
in the bilateral hemisphere. In addition, cortical thinning
was found in the frontal, parietal, temporal, and occipital cortex in the bilateral hemisphere and the right insula.
Larger surface area was found in obese patients compared
to healthy controls in the parietal, temporal, and occipital
cortex in the left hemisphere. The decreased surface area in
obese patients was observed in the left frontal cortex and
right temporal cortex. Increased cortical volumes in obese
patients were shown in the frontal, parietal, temporal, and
occipital cortex in the left hemisphere and the frontal,
temporal, and occipital cortex in the right hemisphere.
Moreover, decreased cortical volumes include the frontal
and occipital cortex in the left hemisphere and the frontal, parietal, temporal, and occipital cortex in the right
hemisphere.
In the previous studies on obesity, several mixed results
have been published with both increased and decreased
cortical structural brain alterations. Compared to normalweight
individuals, obese patients exhibited shrink in
the frontal lobes, anterior cingulate gyrus, hippocampus,
and thalamus [20]. The frontal lobe is involved in the
neural circuitry regulating executive function, cognition,
working memory, and impulse control [21]. The parietal
cortex contributes to attention to learning, encoding,
consolidating, and retrieving memory [22]. A review study
suggests that the temporal lobe which includes two main
structures (e.g., the amygdalae and the hippocampi) has
been reported in the regulation of cognitive and sentimental
functions connected to learning and memory [23].
Moreover, temporal lobe plays a central role in controlling
food intake and body weight. Therefore, the dysregulation
of the temporal lobe can cause functional disturbance in
regulating hunger.
Previous research found that increasing BMI is
connected to cortical thinning in left lateral occipital
cortex and right ventromedial prefrontal cortex [10]. An
fMRI study showed that viewing images of food stimulates
the activation of the occipital gyrus [24]. It has been
demonstrated that increased body weight correlated with
GMV reductions in the occipital lobe [25]. The insular
cortex is composed of three cytoarchitectonic domains
(e.g., anterior ventral agranular, dorsal anterior dysgranular,
and posterior granular) and is identified as the primary
taste and gustation cortex [26]. The anterior insular cortex
is known as the primary hub for processing cognition,
emotion, and sensory stimulation and is responsible for the
regulating appetite and energy balance [27]. Many of these
studies reporting cortical structural alteration findings vary
and the consistency as well as the accuracy of results are
less clear-cut.
Morphological cortical alterations are influenced by
several factors. Cerebral cortex contains intermediate
progenitor cells which are involved in neurogenesis and
reflect laminar thickness, cortical surface area, gyral
patterns [28]. The cerebral cortex also changes depending
on the amount of myelination, cell size, dendritic spines,
synaptic density [29], and neuronal circuits [30]. Surface
area alterations of the cerebral cortex can indicate damages
of white matter tracts due to atrophy of white matter
fibers [31]. Taken together, changes in the cerebral cortex
can indicate changes in neuron number and fundamental
neuropathological support of obese patients.
Previous neuroimaging studies have shown smaller
caudate volumes in adolescents with obesity [32,33]. In
our previous study, we found subcortical GMV reductions
in the bilateral caudate of obese groups compared to
healthy controls [34]. The function of the caudate has been linked to supporting the design and execution of strategies
and behaviors requested for accomplishing complicated
goals [35]. The caudate nucleus is concerned in behavioral
and perceptual processes and forms networks that regulate
cognitive function and emotion [36]. The caudate nucleus
is also known as the key region regulating food intake
through central reward circuits. It is known that improper
regulation of reward circuitry in the brain induces obesity
[37], it is possible that dysfunctional reward circuitry can
be linked to changes in the size of the caudate nucleus.
A previous positron emission tomography (PET) study
documented that because obese subjects have increased
sensitivity to external food stimuli, obese subjects had
altered stimulus-response acquisition. The imbalance of the
brain circuit and reduced cognitive control are the distinct
features of obesity [38].
Obesity is associated with impaired eating control,
reduced cortical gray matter volume, and poor performance
cognitive evaluation [39]. Obesity itself is related to
structural brain atrophy and deficiency of entire and specific
regional brain volume and white matter integrity [40].
Reduced brain volume is likely due to inadequate metabolic
provision. In particular, changes in gray matter or neurons
can be caused by multiple factors such as insufficient
energy supply particularly due to high energy demand of
neurons [41], cellular change, demyelination and neuronal
fiber loss with aging [42]. A study done on rats suggests
that structural plasticity could be affected by the changes in
dendritic morphology [43]. It is important to analyze the
shape differences to identify the exact anatomical location
changes. In addition, knowing regional shape differences is
helpful in interpreting the results of anatomical discoveries
[44]. Measuring brain volume is useful for identifying
neurophysiological changes that occur due to obesity
and linked to other factors such as BMI [45]. Additional
studies regarding these factors will help the understanding
of mechanisms between obesity and volume alterations.
The mechanisms that obesity-related brain connection and
volume alterations are still unclear.
Several limitations should be considered in this study.
First, only male subjects were recruited. A previous study
investigated gender-related differences in obesity, suggesting
that men and women may have different underlying neural
mechanisms [37]. Seconds, the relatively small sample
size limits complex statistical analyses. A larger sample
size would increase statistical significance of this study.
Third, cognitive functioning was not evaluated. Cognitive
function is important for changes in food intake behavior.
The cognitive function is an important for variations in food
intake behavior. Biezonski D, et al. [46] investigated brain
circuit abnormalities and cognitive impairments in subjects
with anorexia nervosa, an eating disorder associated with
underweight and risky eating habits. Therefore, cognitive
performance may be important in regulating food behavior
in obese patients and may influence outcomes.
Conclusion
In conclusion, the present study demonstrates that
obesity is related to structural brain abnormalities in cortical
gray matter regions. Using a surface-based analysis, we
observed distinct differences in cortical thickness, surface
area, and volume in obese patients. The main findings
of the current study show that compared to the healthy
controls, obesity patients had a brain structure involved in
food intake behavior controlling appetite. Therefore, we
provide the evidence that cortical brain structures involved
in food intake behavior are altered in obesity.
Patents
In conclusion, the present study demonstrates that
obesity is related to structural brain abnormalities in cortical
gray matter regions. Using a surface-based analysis, we
observed distinct differences in cortical thickness, surface
area, and volume in obese patients. The main findings
of the current study show that compared to the healthy
controls, obesity patients had a brain structure involved in
food intake behavior controlling appetite. Therefore, we
provide the evidence that cortical brain structures involved
in food intake behavior are altered in obesity.
Funding
This research received no external funding.
Institutional Review Board Statement
This study protocol was approved by the University of
Chungbuk Institutional Review Board (#CBNU-201506-
BMSBBR-059-01), and informed consent was obtained
from each patient.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is unavailable due to privacy or ethical restrictions.
Acknowledgment
This work was supported by the Gachon University
research fund of 2020. (GCU-2020-03020001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Health Organization. Obesity and overweight. 2017.
- Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014:587-591.
Google Scholar, Crossref, Indexed at
- Hall JE, do Carmo JM, da Silva AA, et al. Obesity-induced hypertension: Interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116(6):991-1006.
Google Scholar, Crossref, Indexed at
- Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925-1932.
Google Scholar, Crossref, Indexed at
- Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348(17):1625-1638.
Google Scholar, Crossref, Indexed at
- Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55-92.
Google Scholar, Crossref, Indexed at
- Marqués-Iturria I, Pueyo R, Garolera M, et al. Frontal cortical thinning and subcortical volume reductions in early adulthood obesity. Psychiatry Res Neuroimaging. 2013;214(2):109-115.
Google Scholar, Crossref, Indexed at
- Zhang B, Tian X, Tian D, et al. Altered regional gray matter volume in obese men: A structural MRI study. Front Psychol. 2017;8:125.
Google Scholar, Crossref, Indexed at
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91(5):486-498.
Google Scholar, Crossref, Indexed at
- Medic N, Ziauddeen H, Ersche KD, et al. Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes. 2016;40(7):1177-1182.
Google Scholar, Crossref, Indexed at
- Ronan L, Alexander-Bloch AF, Wagstyl K, et al. Obesity associated with increased brain age from midlife. Neurobiol Aging. 2016;47:63-70.
Google Scholar, Crossref, Indexed at
- Saute RL, Soder RB, Alves Filho JO, et al. Increased brain cortical thickness associated with visceral fat in adolescents. Pediatr Obes. 2018;13(1):74-77.
Google Scholar, Crossref, Indexed at
- Veit R, Kullmann S, Heni M, et al. Reduced cortical thickness associated with visceral fat and BMI. NeuroImage Clin. 2014;6:307-311.
Google Scholar, Crossref, Indexed at
- Widya RL, Roos A, Trompet S, et al. Increased amygdalar and hippocampal volumes in elderly obese individuals with or at risk of cardiovascular disease. Am J Clin Nutr. 2011;93(6):1190-1195.
Google Scholar, Crossref, Indexed at
- Debette S, Wolf C, Lambert JC, et al. Abdominal obesity and lower gray matter volume: A Mendelian randomization study. Neurobiol Aging. 2014;35(2):378-386.
Google Scholar, Crossref, Indexed at
- Pannacciulli N, Del Parigi A, Chen K, et al. Brain abnormalities in human obesity: A voxel-based morphometric study. Neuroimage. 2006;31(4):1419-1425.
Google Scholar, Crossref, Indexed at
- Brooks SJ, Benedict C, Burgos J, et al. Late-life obesity is associated with smaller global and regional gray matter volumes: A voxel-based morphometric study. Int J Obes. 2013;37(2):230-236.
Google Scholar, Crossref, Indexed at
- Rorden C, Karnath HO, Bonilha L. Improving lesion-symptom mapping. J Cogn Neurosci. 2007;19(7):1081-1088.
Google Scholar, Crossref, Indexed at
- Nair VA, Beniwal-Patel P, Mbah I, et al. Structural imaging changes and behavioral correlates in patients with Crohn’s Disease in remission. Front Hum Neurosci. 2016;10:460.
Google Scholar, Crossref, Indexed at
- Raji CA, Ho AJ, Parikshak NN, et al. Brain structure and obesity. Hum Brain Mapp. 2010;31(3):353-364.
Google Scholar, Crossref, Indexed at
- Johnson SB, Blum RW, Giedd JN. Adolescent maturity and the brain: The promise and pitfalls of neuroscience research in adolescent health policy. J Adolesc Health. 2009;45(3):216-221.
Google Scholar, Crossref, Indexed at
- Schiffino FL, Zhou V, Holland PC. Posterior parietal cortex is critical for the encoding, consolidation, and retrieval of a memory that guides attention for learning. Eur J Neurosci. 2014;39(4):640-649.
Google Scholar, Crossref, Indexed at
- Coppin G. The anterior medial temporal lobes: Their role in food intake and body weight regulation. Physiol Behav. 2016;167:60-70.
Google Scholar, Crossref, Indexed at
- Gearhardt AN, Yokum S, Stice E, et al. Relation of obesity to neural activation in response to food commercials. Soc Cogn Affect Neurosci. 2014;9(7):932-938.
Google Scholar, Crossref, Indexed at
- Bond DJ, Ha TH, Lang DJ, et al. Body mass index–related regional gray and white matter volume reductions in first-episode mania patients. Biol Psychiatry. 2014;76(2):138-145.
Google Scholar, Crossref, Indexed at
- Gallay DS, Gallay MN, Jeanmonod D, et al. The insula of Reil revisited: Multiarchitectonic organization in macaque monkeys. Cereb Cortex. 2012;22(1):175-190.
Google Scholar, Crossref, Indexed at
- Frank S, Kullmann S, Veit R. Food related processes in the insular cortex. Front Hum Neurosci. 2013;7:499.
Google Scholar, Crossref, Indexed at
- Pontious A, Kowalczyk T, Englund C, et al. Role of intermediate progenitor cells in cerebral cortex development. Dev Neurosci. 2008;30(1-3):24-32.
Google Scholar, Crossref, Indexed at
- Sowell ER, Peterson BS, Thompson PM, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6(3):309-315.
Google Scholar, Crossref, Indexed at
- Cesa R, Scelfo B, Strata P. Activity-dependent presynaptic and postsynaptic structural plasticity in the mature cerebellum. J Neurosci. 2007;27(17):4603-4611.
Google Scholar, Crossref, Indexed at
- Fung G, Deng Y, Zhao Q, et al. Distinguishing bipolar and major depressive disorders by brain structural morphometry: A pilot study. BMC Psychiatry. 2015;15:1-2.
Google Scholar, Crossref, Indexed at
- Rofey DL, Arslanian SA, El Nokali NE, et al. Brain volume and white matter in youth with type 2 diabetes compared to obese and normal weight, non‐diabetic peers: A pilot study. Int J Dev Neurosci. 2015;46(1):88-91.
Google Scholar, Crossref, Indexed at
- Nouwen A, Chambers A, Chechlacz M, et al. Microstructural abnormalities in white and gray matter in obese adolescents with and without type 2 diabetes. NeuroImage Clin. 2017;16:43-51.
Google Scholar, Crossref, Indexed at
- Kim EB, Baek HM. Volumetric analysis of subcortical structures in obese at 3T. J Magn. 2019;24(2):254-261.
Google Scholar, Crossref, Indexed at
- Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol. 2008;86(3):141-155.
Google Scholar, Crossref, Indexed at
- Robinson JL, Laird AR, Glahn DC, et al. The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60(1):117-129.
Google Scholar, Crossref, Indexed at
- Taghva A, Corrigan JD, Rezai AR. Obesity and brain addiction circuitry: Implications for deep brain stimulation. Neurosurg. 2012;71(2):224-238.
Google Scholar, Crossref, Indexed at
- Nummenmaa L, Hirvonen J, Hannukainen JC, et al. Dorsal striatum and its limbic connectivity mediate abnormal anticipatory reward processing in obesity. PloS One. 2012;7(2):e31089.
Google Scholar, Crossref, Indexed at
- Maayan L, Hoogendoorn C, Sweat V, et al. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obes. 2011 Jul;19(7):1382-7.
Google Scholar, Crossref, Indexed at
- Driscoll I, Gaussoin SA, Wassertheil-Smoller S, et al. Obesity and structural brain integrity in older women: The women’s health initiative magnetic resonance imaging study. J Gerontol Biol Sci Med Sci. 2016;71(9):1216-1222.
Google Scholar, Crossref, Indexed at
- Dommes E, Georgiewa P, Klingebiel R. Grey matter volume differences in obese as compared to normal-weight individuals: A voxel-based morphometric study. Arch Euromed. 2013;3(2):11-16.
Google Scholar
- Sowell ER, Thompson PM, Toga AW. Mapping changes in the human cortex throughout the span of life. Neurosci. 2004;10(4):372-392.
Google Scholar, Crossref, Indexed at
- Cazorla M, Shegda M, Ramesh B, et al. Striatal D2 receptors regulate dendritic morphology of medium spiny neurons via Kir2 channels. J Neurosci. 2012;32(7):2398-2409.
Google Scholar, Crossref, Indexed at
- Hermesdorf M, Sundermann B, Rawal R, et al. Lack of association between shape and volume of subcortical brain structures and restless legs syndrome. Front Neurol. 2018;9:355.
Google Scholar, Crossref, Indexed at
- Wang H, Wen B, Cheng J, et al. Brain structural differences between normal and obese adults and their links with lack of perseverance, negative urgency, and sensation seeking. Sci Rep. 2017;7(1):40595.
Google Scholar, Crossref, Indexed at
- Biezonski D, Cha J, Steinglass J, et al. Evidence for thalamocortical circuit abnormalities and associated cognitive dysfunctions in underweight individuals with anorexia nervosa. Neuropsychopharmacol. 2016;41(6):1560-1568.
Google Scholar, Crossref, Indexed at









