Jacob L. Ransom1 and Nikoletta Carayannopoulos2*
1Department of Orthopaedic Surgery, University of Texas Medical Branch at Galveston, Galveston, USA
2Department of Orthopaedic Surgery and Rehabilitation, University of Texas Medical Branch at Galveston, Galveston, Texas, USA
*Corresponding Author: Nikoletta Carayannopoulos
University of Texas Medical Branch at Galveston, Galveston, Texas, USA
Tel: 4095027177
E-mail: greekniki@yahoo.com
Received date: November 02, 2016; Accepted date: December 08, 2016; Published date: December 13, 2016
Citation: Ranson JL, Carayannopoulos N. Cupriavidus pauculus Osteomyelitis and Secondary Septic Arthritis with Hemodialysis Origins: A Case Study. Transl Biomed. 2016, 7: 4. doi: 10.2167/2172-0479.100095
Keywords
Septic arthritis; Hemodialysis; Cupriavidus pauculus; Bone biopsies
Introduction
Chronic osteomyelitis is a debilitating disease that requires a combination of surgical intervention and antibiotic therapy versus antibiotic therapy alone [1]. Sources of infection typically include spread from contiguous soft tissue, trauma, surgery and hematogeous spread [2]. Hematogenous osteomyelitis may be associated with any event of transient bacteremia with common origins including intravenous drug abuse, indwelling catheterization, and other infectious foci [3]. The condition of the host, and the invading organism are related. In healthy adults, Staphylococcus aureus is the number one causative organism whereas Aspergillus, Candida, and Mycobacteria are more commonly isolated from immunocompromised patients [1]. One potential complication of osteomyelitis, as well as a medical emergency, is seeding to the adjacent joint causing septic arthritis [4]. We report a case of chronic osteomyelitis of the distal tibia and talar dome with subsequent septic arthritis of the ankle joint in an immunocompromised patient and the first case of osteomyelitis from Cupriavidus pauculus.
Case Presentation
A 28-year-old female presented to our emergency department on 02/27/2015 with complaints of acute right ankle pain, tachycardia, hypotension, and nausea during fluid removal at dialysis. She has an extensive past medical history including chronic glomerulonephritis requiring kidney transplant with subsequent rejection and multiple foci of osteonecrosis from corticosteroid use. Radiographs of the right ankle revealed no bony abnormalities with prominent soft tissues over the lateral ankle. Physical exam disclosed mild swelling over the lateral malleolus with tenderness but no deficits in range of motion. Normal saline was administered intravenously due to suspected excess volume removal at dialysis. Her right ankle pain was initially diagnosed as acute gout after both labs and radiology results were unremarkable and 0.6 mg per day oral Colchicine was prescribed and she was discharged.
Two weeks later, she presented to her primary care provider for pain and swelling in the right ankle that was unresponsive to Colchicine. She received an injection of 1.5 ml kenalog (40 mg/ml) in her right ankle.
The patient followed up one week later claiming significant pain improvement following the injection. However, she claimed to have hit her right ankle on the bed the day before her visit and complained of new pain and swelling. Inflammation and potential soft tissue damage was suspected so a referral to orthopaedics was scheduled for two days later. However, two days later, she was admitted for severe right ankle pain. She had full range of motion and no difficulty ambulating. Radiographs were obtained and a poorly defined intramedullary lucency was witnessed at the distal tibia owing to potential osteomyelitis. Tibiotalar joint space loss was also noted, suggesting septic arthritis. Intravenous administration of vancomycin 750 mg and gentamycin 100 mg was initiated pending an MRI. The MRI suggested osteomyelitis of the distal tibial diametaphysis with tibial talar septic arthritis. Tenosynovitis of the plantar flexor tendon group was also reported. Patient was examined by orthopaedics and was scheduled for an emergent right ankle arthrotomy and subsequent drainage. A lateral arthrotomy of the right ankle was performed and 10 cc of pus was extracted from the joint and was sent for aerobic, anaerobic, AFB, and fungal cultures. The arthrotomy sites were copiously irrigated with 4.5 L of normal saline and the wound was closed in layers with subsequent placement of a splint.
The patient continued to receive vancomycin 400 mg IV piggyback and gentamycin 50 mg IV piggyback with dialysis. Wound gram stain was negative. Culture showed gram negative rods. Our patient maintained an afebrile status with normal CBC and stated that her pain was significantly reduced. She was discharged on IV gentamycin for two weeks during dialysis and 500 mg oral levaquin every 48 hours for five weeks. The patient remained asymptomatic for the next 8 months. During this time, definitive treatment options including amputation or surgical debridement were discussed with our patient but she was not amenable to these suggestions.
Three rare gram negative species grew from her intraoperative cultures following discharge; Roseomonas gilardi, Cupriavidus pauculus, and Pandoraea spp. Ceftriaxone was subsequently added to her current regimen of oral levaquin and IV gentamicin and would be administered during dialysis with the gentamicin.
One month later, MRI revealed no change in the right tibia osteomyelitis despite attempted antibiotic treatment although the patient was not experiencing any symptomology. Again, definitive treatment options were discussed with her but were rejected at the time.
Approximately 8 months after initial surgery, our patient was admitted for intermittent right ankle pain for the last three weeks with inability to ambulate after periods of standing. She also confirmed fever and chills while at home. MRI suggested worsening osteomyelitis of her tibia and talus. She was diagnosed with septic arthritis of the right ankle. Imipenem-cilastatin 500 mg BID IV piggyback was started and the patient was taken to the operating room for a right ankle medial/lateral arthrotomy with subsequent bone biopsy of the distal tibia. No purulent fluid was found in the joint space. Cortical and cancellous bone samples were obtained and sent for aerobic, anaerobic, AFB, and fungal cultures and the arthrotomy sites were copiously irrigated with 6 L of normal saline. Bone biopsies revealed an infection by Cupriavidus pauculus. A peripherally inserted central catheter was placed for our patient to undergo 6 weeks of imipenem-cilastatin 250 mg BID based on this organism’s sensitivity. Definitive treatment by amputation or surgical debridement with impregnation of an antibiotic spacer were again discussed with our patient at length but she desired a second opinion before deciding on treatment course. In the interim, the patient was in need of a kidney transplant but had been taken off the transplant list in the setting of persistent infection. She opted for surgical debridement and decided to forego the recommended treatment for amputation. She decided to undergo initial surgical debridement with antibiotic spacer placement the following week. Samples from proximal and distal margins would be cultured and, if clear, the antibiotic spacer would be replaced and would be internally fixed. Given the patient’s immunocompromised status and large bone defect from removal of the osteomyelitis, she understood that risk for recurrent infection, morbidity bone grafting and failure would be high so she opted for retention of the antibiotic cement with plate fixation to be her definitive construct that would allow her to be placed back on the transplant list. Also, her functional mobility with her chronic kidney condition has always been limited to household and short-distance ambulation with a cane which would put limited strain on her fixation construct.
The patient was taken to the operating room and the right tibia and talar dome were resected with a sagittal saw and bone samples were taken from proximal and distal ends to determine if the margins were liberated from infection. The wound was thoroughly washed out with 9 L of isotonic saline solution. An antibiotic cement spacer of 1.0 g vancomycin and 3.6 g tobramycin was placed in the area of debridement and the wound was closed. The patient left the operating room with a splint on her right leg. Bone margin samples were found to have no signs of inflammation or persistent osteomyelitis. Four days later, the patient was brought back to the operating room for replacement of the spacer, a wash out with isotonic saline solution, and open reduction internal fixation (ORIF). Final radiographs from the ORIF are shown in Figure 1. Our patient recovered from surgery without complications and is currently 5 months out from her definitive procedure. She is participating in a physical therapy regimen and awaiting kidney transplant.
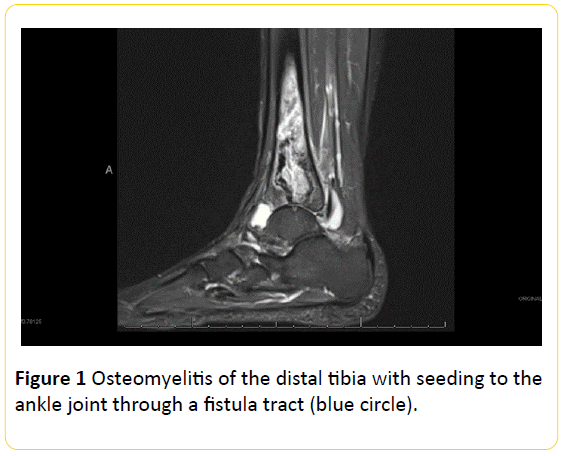
Figure 1: Osteomyelitis of the distal tibia with seeding to the ankle joint through a fistula tract (blue circle).
Discussion
Cupriavidus pauculus is an aerobic, motile, gram negative bacillus typically isolated from water and soil. Taxonomically, this organism has been formally known as Centers for Disease Control (CDC) group IVc-2, which was reclassified as Ralstonia paucula sp. nov. [5] and later assigned to Wautersia paucula [6]. The organism has since been more accurately categorized under the genus Cupriavidus [7]. Historically, C. pauculus has been described as rarely pathogenic [5-8]. To our knowledge, this is the first reported case of osteomyelitis caused by Cupriavidus pauculus.
A study by Balada-Llasat, et al. [9] demonstrated a pseudooutbreak of C. pauculus in a clinic as a result of sampling methods that included the use of contaminated water [9]. Likewise, Tasbakan et al. [10] reports a case of ventilator associated pneumonia caused by C. pauculus in which imipenem therapy 500 mg (4 × 1) for 14 days eradicated the infection [10]. This is in contrast to what we observed with our use of the imipenem.
Initial cultures had shown infection by C. pauculus, R. gilardi, and Pandorea spp. Cultures after the second operative debridement only showed C. pauculus suggesting that R. gilardi and Pandorea spp. were eradicated with the aforementioned 10-week regimen of gentamicin, levaquin, and ceftriaxone. Thxis was the reason we chose to focus on Cupriavidus pauculus. Given that this organism has been isolated from contaminated water and documented to be rarely pathogenic, [5,6,8] we hypothesize that this patient was exposed to transient bacteremia with the source being contaminated water that was used at the dialysis unit. At the time the cultures were received, the patient was notified of our concerns and changed dialysis units immediately.
Chronic osteomyelitis is a severe infection that warrants prompt surgical and antibiotic therapy. Virtually all pathogenic organisms can cause osteomyelitis; however, Staphylococcus aureus is the most common infective agent [1,11,12]. Sources may include direct inoculation through trauma or surgery, spread from a contiguous soft tissue focus, or by hematogenous routes [2]. After delivery, bacteria will bind to the bone and generate a biofilm to proliferate [13]. A host inflammatory process then ensues whereby neutrophilic phagocytosis begins. Subsequent reactive species and inflammatory cytokines are generated from the host’s response to the bacterial matrix [13]. Ultimately this process results in the destruction and resorption of bone [11,12]. Obliteration of the Haversian and Volkmann canals will ultimately compromise blood flow to the focus and cause the formation of an avascular sequestrum [12]. The formation of these sequestra account for the difficulty in treating this disease due to the poor delivery of medications [11].
It is known that septic arthritis is a complication of adjacent osteomyelitis [4,14,15]. However, this process is not very common in adults as is the case of our patient [12]. Septic arthritis is a medical emergency that requires rapid surgical management with systemic antibiotic treatment [14]. Clinically, septic arthritis becomes difficult to treat if the cause is an unknown area of quiescent osteomyelitis because recurrent episodes are more likely [16]. This was demonstrated in the case of our patient. The osteomyelitis focus remained unseen on radiographic studies and was therefore only discovered due to an emergent case of septic arthritis. Once resolved, she developed a subsequent case 8 months later.
One pertinent risk factor for osteomyelitis is immunosuppression [17]. Patients with an abnormal immune response may not demonstrate the normal inflammatory response that is seen when bacteria bind to the bone. Therefore, immunosuppressed patients may not wield a typical presentation thereby hindering the initial diagnosis of the patient [18]. This was validated in our patient who was originally diagnosed with gout with no radiological or clinical signs or symptoms of infection. Charity and Foukas [18] demonstrated a similar scenario and emphasized the importance of carefully investigating bone pain in immunocompromised patients. Aberrant symptomatology was also noted when our patient developed septic arthritis. She endorsed episodes of pain but denied difficulty ambulating. Physical exam had also revealed full range of motion in the ankle joint. These deviations are also attributed to her immunocompromised state.
Aside from immunosuppression, extensive corticosteroid use for multiple years resulted in the development of osteonecrosis in various regions including her hip and right distal tibia. We believe that these areas of necrosis provided the proper environment for bacterial seeding. Based on the MRI in Figure 2, our hypothesis is that, hematogenous infection began in the distal tibia, in an area of previous necrosis, and seeded into the ankle joint through a fistula tract thereby facilitating the development of septic arthritis.
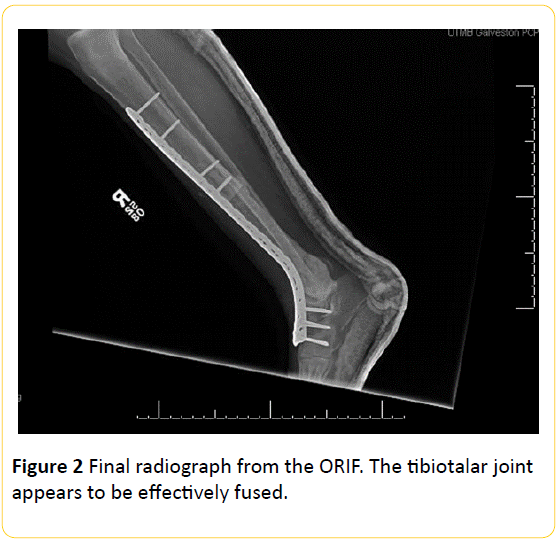
Figure 2: Final radiograph from the ORIF. The tibiotalar joint appears to be effectively fused.
Although multiple modalities of treatment are available for patients with chronic osteomyelitis, the only definitive cure for the disease is excision of the affected area and in the case of joint involvement with progression of infection distal and proximal joint-amputation. Because of the recurring episodes of septic arthritis and resistance of the infection to imipenem, we discussed the recommended treatment of a below-theknee amputation with our patient but she was not amenable to this option. As a result, debridement followed by dead space management was pursued. Typically for larger defects, as in this case, bone grafting or bone transport mechanisms are first line [11]. However, in this case of our patient, we chose to manage dead space with an antibiotic cement spacer. The reason for this was due to our patient’s low lifestyle demands that would not include any strenuous physical activity. In patients seeking regular activity level, this method of care would not be justified. It is also necessary for our patient to use an ankle-foot-orthosis (AFO) to prevent too much ankle mobility which could possibly loosen the screw fixation in her foot.
In conclusion, chronic osteomyelitis is a cumbersome disease which requires surgical intervention. Here, we present a case of osteomyelitis of the distal tibia and talar dome in an immunocompromised patient on hemodialysis. The cultured organism was a rarely pathologic gram negative bacillus, Cupriavidus pauculus. The infected portions were debrided and an antibiotic spacer was placed and fixed. This surgery was an alternative to amputation and was amenable to this patient because of her low lifestyle demands. Our patient experienced no complications during surgery and is progressing with her physical therapy regimen and has been placed back on the transplant list.
17728
References
- Calhoun JH, Manring MM, Shirtliff M (2009) Osteomyelitis of the long bones. Semin Plast Surg 23: 59-72.
- Goldenberg DL, Reed JI (1985) Bacterial arthritis. N Engl J Med 312: 764-771.
- Vandamme P, Goris J, Coenye T, Hoste B, Janssens D, et al. (1999) Assignment of Centers for Disease Control Group IVc-2 to the genus Ralstonia as Ralstonia paucula sp. nov. Int J Syst Bacteriol 49: 663-669.
- Vaneechoutte M, Kämpfer P, De Baere T, Falsen E, Verschraegen G (2004) Wautersia gen. nov., a novel genus accommodating the phylogenetic lineage including Ralstonia eutropha and related species, and proposal of Ralstonia [Pseudomonas] syzygii (Roberts et al. 1990) comb. nov. Int J Syst Evol Microbiol 54: 317-327.
- Vandamme P, Coenye T (2004) Taxonomy of the genus Cupriavidus: a tale of lost and found. Int J Syst Evol Microbiol 54: 2285-2289.
- Uzodi AS, Schears GJ, Neal JR, Henry NK (2014) Cupriavidus pauculus bacteremia in a child on extracorporeal membrane oxygenation. ASAIO J 60: 740-741.
- Balada-Llasat JM, Elkins C, Swyers L, Bannerman T, Pancholi P (2010) Pseudo-outbreak of Cupriavidus pauculus infection at an outpatient clinic related to rinsing culturette swabs in tap water. J Clin Microbiol 48: 2645-2647.
- Taøbakan MS, Yamazhan T, Aydemir S, BacakoÃlu F (2010) A case of ventilator-associated pneumonia caused by Cupriavidus pauculus. Mikrobiyol Bul 44: 127-131.
- Parsons B, Strauss E (2004) Surgical management of chronic osteomyelitis. Am J Surg 188: 57-66.
- Kumar V, Abbas AK, Aster JC (2015) Robbins and Cotran pathologic basis of disease. (9thedn), Philadelphia, PA: Elsevier/Saunders.
- Beck-Broichsitter BE, Smeets R, Heiland M (2015) Current concepts in pathogenesis of acute and chronic osteomyelitis. Curr Opin Infect Dis 28: 240-245.
- Zalavras CG, Dellamaggiora R, Patzakis MJ, Zachos V, Holtom PD (2006) Recalcitrant septic knee arthritis due to adjacent osteomyelitis in adults. Clin Orthop Relat Res 451: 38-41.
- Atcheson SG, Ward JR (1978) Acute hematogenous osteomyelitis progressing to septic synovitis and eventual pyarthrosis. The vascular pathway. Arthritis Rheum 21: 968-971.
- Jackson MA, Burry VF, Olson LC (1992) Pyogenic arthritis associated with adjacent osteomyelitis: identification of the sequela-prone child. Pediatr Infect Dis J 11: 9-13.
- Khan K, Wozniak SE, Mehrabi E, Giannone AL, Dave M (2015) Sternoclavicular osteomyelitis in an immunosuppressed patient: A case report and review of the literature. Am J Case Rep 16: 908-911.
- Charity RM, Foukas AF (2005) Osteomyelitis and secondary septic arthritis caused by Sphingomonas paucimobilis. Infection 33: 93-95.







