Wellington Luiz de Oliveira da Rosa1, Simone Gomes Dias de Oliveira2, Caroline Huber Rosa3, Adriana Fernandes da Silva4, Rafael Guerra Lund3 and Evandro Piva3*
1Post-Graduate Program in Dentistry, Federal University of Pelotas, Pelotas, RS, Brazil
2Department of Dental Materials, School of Dentistry, Federal University of Vales do Jequitinhonha e Mucuri, Diamantina, MG, Brazil
3Federal University of Pelotas, Pelotas, Brazil
4Department of Restorative Dentistry, Federal University of Pelotas, Pelotas, Brazil
Corresponding Author:
Dr. Evandro Piva
Department of Restorative Dentistry., Federal University of Pelotas.
Gonçalves Chaves st., 457., room 504., Centro 96015- 560., Pelotas., RS., Brazil
Tel: +55 53 3225-6741
Fax: +55 53 3225-6741
E-mail: evpiva@pq.cnpq.br
Citation: de Oliveira da Rosa WL, de Oliveira SGD, Rosa CH, et al. Current Trends and Future Perspectives in the Development of Denture Adhesives: An Overview Based on Technological Monitoring Process and Systematic Review. J Biomedical Sci. 2015, 4:1. doi:10.4172/2254-609X.10003
Copyright: © 2015 de Oliveira da Rosa WL. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Key words
Dental prosthesis, Dentures, Denture adhesives, Dental materials, Systematic review.
Introduction
The prevalence of complete tooth loss has decreased over the last decade, but tooth loss remains a major disease worldwide, especially among older adults [1]. Currently the use of implants are several suitable alternatives can be explored for oral rehabilitation of these patients, however conventional dentures are the most important treatment option, mainly in developing countries, such as Brazil, due to affordability of treatment [2]. Among the objectives to be achieved when making a conventional denture, retention and stability issues of great interest on the part of its, since they are directly related to your comfort and safety [3, 4]. Denture adhesives are commercially available dental materials that have long been recognized as a useful adjunct to improve denture retention, stability and function [5-7]. The value of retention obtained with adhesives can be more than twice as high when compared with that of dentures used without these products [8].
Although some dentists are reluctant to indicate these materials, because it could imply that the dentures were incorrectly fabricated and present lack of retention and stability, the use of these materials may be a therapeutic and efficient procedure for the preparation and subsequent use of total dental prosthesis [9-11]. Denture adhesives can be useful to perform clinical procedures that demand more stability of the denture base, such as at the time of obtaining maxillo-mandibular relationship and during setting up of the teeth. They can be used in patients with sensitive mucosa, acting as a cushion to reduce tissue irritation resulting from compression such as ulcers and inflammation. Moreover, they also help to increase the stability of temporary and/or immediate dentures [9,12,13].
The American Dental Association first reported the use of denture adhesives in 1935, and the first patent related to this material was issued in the United States in 1913 [9,10]. The materials used in the 19th century were a mixture of vegetable gums forming a material capable of absorbing moisture from saliva. After hydration, the mixture increased in volume, and became a viscous material that enabled the prosthesis to adhere to the buccal mucosa. Usually, their mechanism of action results from increased contact between the tissues and denture, which creates a retentive force between the oral mucosa and the prosthesis [14,15, 7]. The main components in these materials are compounds responsible for adhesive properties (karaya gum, tragacanth, acacia, pectin, gelatin, methyl cellulose, polyethylene oxide, acrylamide, polyvinyl chloride), antimicrobial agents (sodium borate, hexachlorophene, methyl paraben), additives, wetting and plasticizing agents [9]. Nowadays these materials are available in formulations such as powders, pastes or creams for soluble adhesives, and strips or cushions for insoluble materials [14,16,17,12,18,13]. The soluble group presents synthetic agents, which depend on the chemical properties of one or more active ingredients that increase its volume from 50% to 150% and become viscous and sticky in the presence of water or saliva, filling the spaces between the base of prosthesis and the supporting tissues. The active ingredients are a mixture of salts of polymers with different degrees of solubility in water, which are designed to produce short and long acting adhesive. The carboxymethylcellulose (CMC) and methylcellulose, and the polyvinylether (PVM-MA) are examples of short and longacting salts, respectively. The CMC salts provide a strong initial retention, however due to its high solubility, they dissolve rapidly, losing its effectiveness within a short period. The PVMMA salts, however, have a low solubility, which takes longer to activate them and have a longer period of action. Subsequently, to improve their effectiveness it was added calcium and zinc salts in denture adhesives formulation. Despite the fact that the tape composition varies between trademarks, they all include essentially a manufactured blade impregnated with active component water based. Examples of adhesive ingredients include sodium alginate or polymer of ethylene oxide, which become sticky when activated by the saliva, and the difference between them is the thickness of the blade [19].
The use of methods capable of mapping scientific and technological developments (i.e. “Technology monitoring”, “Technology intelligence” and “Technology forecasting””) may enable opportunities to be identified for current and future technological scenarios [20,21]. There is a large market for this technological sector, and institutions and companies should conduct research and development (R and D) activities in areas presenting market demand [22-25]. However, it is not easy to orient their strategies and use them to their own benefit [23, 26]. Technological information contained in patents can be a fundamental instrument for strategic planning, since they contain a detailed description of the invention, which is often not available in another document [22,27,28]. It is estimated that almost 80% of all technological information can be found only in patent publications [20,29]. Technology management is a set of management disciplines that allows organizations to make a better use of their capital to maintain their competitive edge [30]. Patent data analysis can also be used to help to analyze industry trends, to identify business opportunities and encourage innovation [31,32]. Other important applications include the possibility of conception of scientific-technological projects to obtain financing, the development of new materials, and establishment of partnerships between universities and companies [31].
In competitive business environments, patent intelligence, the transformation of content found in patents into technical, business, and legal insight, is getting attention as a tool to aid efforts to secure competitive advantages [25]. Furthermore, the analysis of scientific and technological information can allow the identification of opportunities for new product, process or device development [33]. Thus, the aim of this study was to systematically review articles and patents related to denture adhesives in order to use as a strategic tool for prospecting in this technological sector.
Methods
Data sources and search strategy
This systematic review was performed according to the PRISMA statement [34]. The search strategy included the following seven databases: MedLine (PubMed), Web of Science, Lilacs, Ibecs, Cochrane Library, Scielo and Scopus. In addition, the search and analysis of patent applications was conducted by the online system Questel Orbit (Paris, France), which contains patent data on over 90 authorities. The following addition patent databases were screened: USPTO (United States Patents and Trademark Office), EPO (European Patent Office), JPO (Japan Patent Office), INPI (National Institute of Intellectual Property of Brazil), Derwent Innovations Index and Patentscope. The search strategy used in PubMed is listed in Table 1, and in all other databases the same keywords were used. The references cited in the papers included were also checked; and the cited articles were also tracked using SCOPUS citation tools.
| Search |
Terms |
| #4 |
Search #1 AND #2 AND #3 |
| #3 |
Search Dental Materials OR Materials, Dental OR Dental Material OR Material, Dental OR Oral medicine OR Stomatology OR Medicine, Oral OR Dentistry OR Odontology |
| #2 |
Search (Dental prosthesis retention OR retention, dental prosthesis OR prosthesis retention, dental OR denture detention OR retention, denture OR denture stability OR stability, denture OR fixative denture OR denture, fixative OR dental prosthesis fixative OR fixative, dental prosthesis OR adhesive denture OR denture, adhesive OR dental prosthesis adhesive OR adhesive, dental prosthesis[Title/Abstract]) |
| #1 |
Search (Dental Prosthesis OR Prostheses, Dental OR Dental Prostheses OR Prosthesis, Dental OR Dentures[Title/Abstract]) |
Table 1 Search strategy used in PubMed (MedLine).
Furthermore, a patent search was also made using International Patent Classification (IPC) with the following codes: A61K-6/00 (preparations to dentistry), A61C-8/00 (means to fix dentures), A61C-13/00 (dentures), A61C-13/23 (adhesive films). Just as in most applications, each patent may submit more than one CIP. This technology and its applications are related to different areas of science and technology, such as dentistry, chemistry or pharmacology, and it can interfere with them. The aim of identifying these codes is to create a specific tool for search and retrieval of documents.
Afterwards the duplicates were removed using the program EndNote x7 (Thompson Reuters, Philadelphia, PA, USA). Reviewers selected documents of interest independently after reading the title and abstract. The inclusion criteria consisted of selecting only patents and articles related to denture adhesives published between January 1960 and April 2014. English, Spanish and Portuguese documents were screened. As exclusion criteria, it was not included literature reviews, documents not related to denture adhesives, and articles or patents related to dental adhesives with applications differing from assisting the retention and stability of dentures.
Data extraction
The title and abstract of each article identified were screened by two independent reviewers (WLOR and CHR) to determine whether the article and patent should be further considered for inclusion. After the screening, the articles included were read in full in order to extract the relevant data. Two reviewers, who were not blinded to the publication authors or inventors, independently performed the data extractions. Disagreements were resolved by discussion to reach a consensus, or through a third reviewer (EP).
Data were extracted by the Microsoft Office Excel 2013 software program (Microsoft Corporation, Redmond, Washington, USA) using a standardized form. Reviewers tabulated the data of interest to compose a spreadsheet in Excel format with all the trial documents containing: the title of patents and articles, the applicant or author's names, the inventor's name, the priority date of patents, the publication date of articles, the document status, the International Patent Classification, the types of denture adhesives used, priority countries and the type of study conducted.
Data analysis
Scientific and technological information was analyzed by the Microsoft Office Excel 2013 software program (Microsoft Corporation, Redmond, Washington, USA). In addition, the online system Questel Orbit (Paris, France) was used to analyze the following technological data obtained from patents: number of patents deposited worldwide, main countries with patents deposited, and institutions and companies with patents related to denture adhesives. Two reviewers (WLOR e CHR), who received training in these software programs, conducted the analysis independently.
Results
The search initially retrieved 156 patents, with 59 being excluded after reading the title and abstract (Figure 1). Out of 97 patents selected, 15 were excluded because they did not fit the eligibility criteria, since they were not related to denture adhesives. A total of 82 patents were included in the analysis. Moreover, in the search of scientific articles, 9,494 were identified initially. After removing the duplicates and reading the title and abstract, 7913 were excluded because were not related to denture adhesives, or were related to dental adhesives with applications differing from assisting the retention and stability of dentures. A total of 81 articles remained and 9 were excluded (4 involved a literature review and 5 were not related to denture adhesives). A total of 72 articles fulfilled all criteria and were included in the analysis.
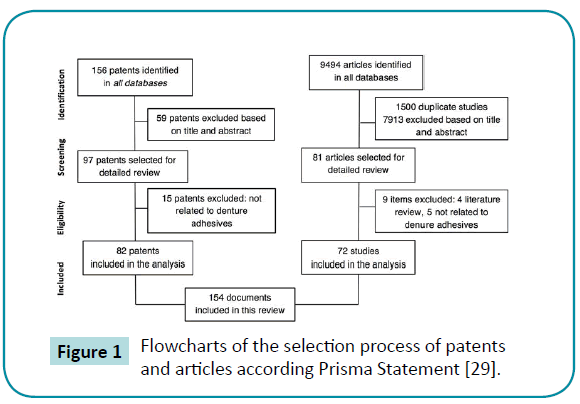
Figure 1: Flowcharts of the selection process of patents and articles according Prisma Statement [29].
Out of the studies included, 56% were in vivo (clinical trials or animal experimentations). There was more presence of the combined or individual use of the type cream/paste and powder (Figure 2). As regards priority patents, 63% and 33% of published articles were from the United States, the country with the most patents deposited in the sector (Figure 3). The American company Procter and Gamble (Cincinnati, Ohio, USA) had the largest number of patents related to denture adhesives (Figure 4). Moreover, there was a remarkable increase in scientific and technological production in the 1990s, as demonstrated in (Figure 5).
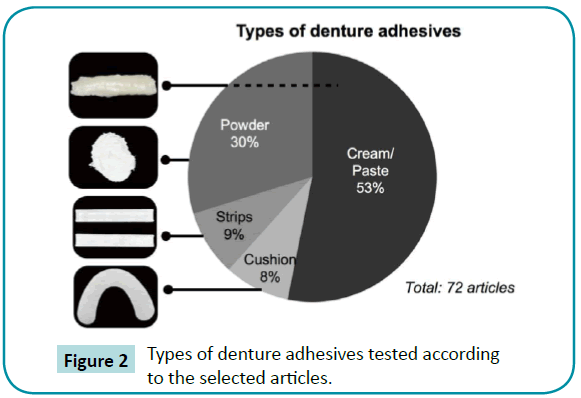
Figure 2: Types of denture adhesives tested according to the selected articles.
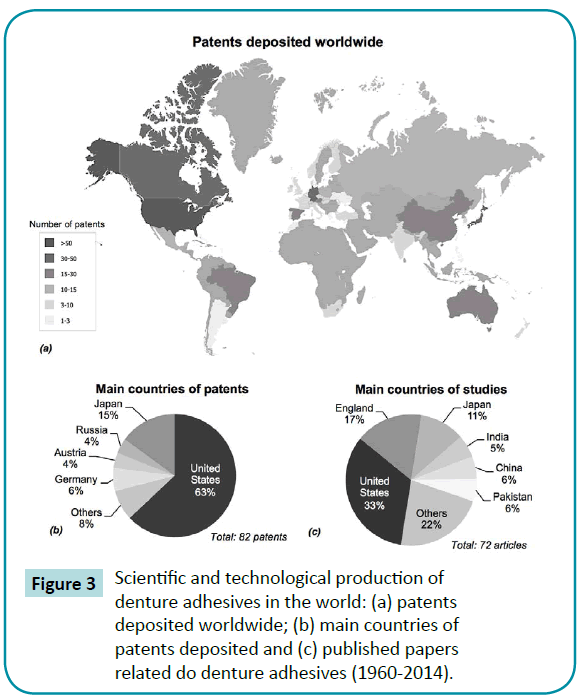
Figure 3: Scientific and technological production of denture adhesives in the world: (a) patents deposited worldwide; (b) main countries of patents deposited and (c) published papers related do denture adhesives (1960-2014).
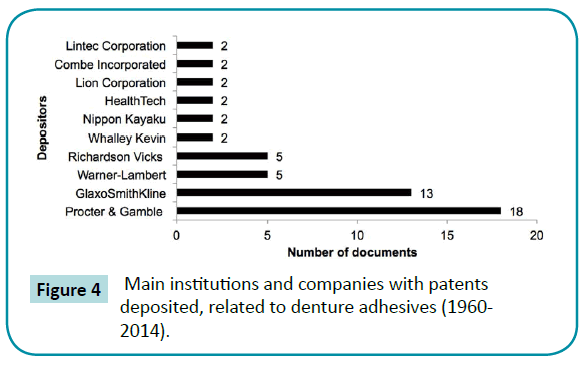
Figure 4: Main institutions and companies with patents deposited, related to denture adhesives (1960-2014).
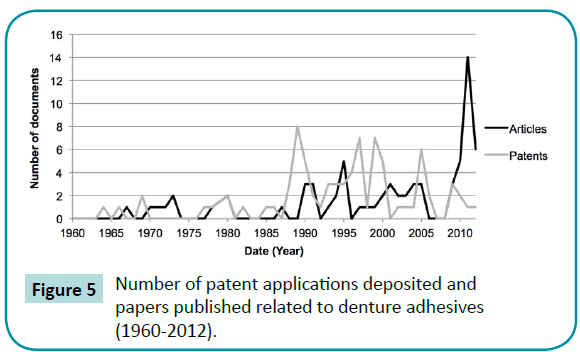
Figure 5: Number of patent applications deposited and papers published related to denture adhesives (1960-2012).
Discussion
This technological monitoring allowed the knowledge available in the denture adhesive sector to be mapped, and it was possible to obtain a scientific and technological overview of these materials. Furthermore, this type of analysis can allow technological prospecting by institutions and companies, which is imperative for scientific and technological innovation [23,35-37]. As demonstrated in (Figure 5), there was a gradual increase in studies related to denture adhesives, with a predominance of patents deposited in the 1990s. Considering that the first patent related to the material dates back to 1913 [9,10], it seems that the interest in the market has intensified over the past 25 years. Furthermore, in the denture adhesive field there is a larger body of technological than scientific information available, which means that only the search and analysis of articles might show a different and limited overview of this technology.
An ideal denture adhesive is described in the literature as being nontoxic, nonirritating, and biocompatible with the oral mucosa and not promoting microbial growth. Furthermore, the product should be odorless, tasteless, and easy to apply and to remove from the tissue-bearing surface of dentures [9,10]. In an endeavor to develop products that satisfied these criteria, the composition of denture adhesives continues to change as the manufacturers try to improve the efficacy of their products. In the 1970s calcium salts were added, and in the 1980s the effectiveness of denture adhesives was improved by adding zinc to the previous formulations. Active ingredients in current formulations can include combined polymethyl vinyl ether-maleic anhydride (PVM-MA) zinc, which are high molecular weight copolymers with adhesive and cohesive properties, and calcium salts with carboxymethylcellulose, a viscosity modifier [38,39,19].
Denture adhesives that contain zinc were recently withdrawn from the market by companies in some countries (i.e. Super Poligrip “Original”; Super Poligrip “Ultra Fresh"; Super Poligrip “Extra Care”, GlaxoSmithKline, London, UK). The presentation in powder form could be more dangerous to patients, since it would be more easily aspirated and could jeopardize the denture wearer’s health. Chronic, excessive ingestion of zinc may result in copper deficiency, which is an established and increasingly recognized cause of neurologic disease [40]. It also may cause bone marrow suppression and polyneuropathy that can result in numbness and paresthesia of the extremities, loss of balance, and walking problems [40,41]. The current trend is the development of zinc-free products (i.e., Super Poligrip “Free”, GlaxoSmithKline, London, UK), in order to try to avoid the side effects of this compound [41].
In the future, the use of additives such as antimicrobial or antifungal agents could gain popularity, especially for use in edentulous patients who are susceptible to diseases such as candidiasis [18].
Strategically, the material can be used as a delivery system for various types of medicines, containing components to help to reduce irritation and inflammation of the oral mucosa adjacent to the denture. Furthermore, a current challenge is the development of denture adhesives with properties that do not allow the adhesion of food or even bacterial plaque, so that they can be used for longer periods of time without the necessity of daily exchanges. Moreover, it was found that the manufacturers of current materials have not yet found a way to deal with the challenge of maintaining their properties for longer periods without jeopardizing their odorless and tasteless qualities, or their inhibition of bacterial growth.
It was estimated that between 15% and 33% of patients with dentures were users of these adhesives [42,43]. In the USA alone, the use of complete dentures was projected to increase to 61.0 million in 2020 [1]. It was also reported that about 5 million Americans are denture adhesives users [19]. Besides, previous studies showed a denture adhesive usage among denture wearers of 7% in Australia [42], 20% in Netherlands and 26% in Greece [44]. With regard to the technological development of the sector, the majority of scientific articles (33%) and patent documents (63% of priority) came from the United States. Furthermore, according to National Survey of Oral Health (Projeto SB Brasil, 2010), almost 25% of Brazilian population wear dentures. There is a large market value for the development of these materials, which is not being properly exploited by many countries. For example, Brazil showed no patent or article published in this sector. The patents filed in these underdeveloped countries are mostly foreign-owned. Moreover, countries can use patented technologies to obtain competitive advantages, since the strategic use of intellectual property has become a vital tool of corporate strategy in the global economy [31]. The country with the highest number of deposited patents related to denture adhesives was the United States (Figure 3). This information can be of great value to companies and research centers that aim to protect new adhesives, since it allows them to identify important markets for this technology [45].
Furthermore, cooperation can be decisive for companies or institutions that need to use limited resources in the most efficient way [31,35,]. The analysis of the main companies that have worked on developing this material (Figure 4) indicates that Procter and Gamble (Cincinnati, Ohio, USA) and GlaxoSmithKline (London, UK) had the highest number of patent deposits observed, with 18 and 13 respectively. This type of information can be useful to universities, research centers or independent researchers seeking partnerships to develop new adhesives. The benefits of this type of cooperation includes the division of costs for developing new products; shortened lead times; and that each company can contribute with its own competence [31,45].
The patent document is an essential source of information for technological analysis, considering the wide variety of content available only in this type of document [46,47]. However, there are limitations to using patent data as an indicator of technological development: not all inventions meet the patentability standards and inventors can rely on secrecy or other appropriate means to protect their inventions [23,48]. Furthermore, there is a time lag of at least 18 months between the first patent filing and the patent publication [23]. Because of this, the most recent patents included in this study were deposited up to October 2012. Moreover, each patent office uses a different tool that allows the recovery of documents, which makes it very difficult to collect and find interesting information [22,45]. Therefore, there is a need for obtaining licenses to software programs that facilitate technological monitoring by institutions and companies, as Questel Orbit (Paris, France) or VantagePoint (Search Technology, Inc., Norcross, GA, USA).
From this review it was possible to obtain a scientific and technological overview of the field of denture adhesives. By combining and analyzing scientific and technological information, the design of this study can provide strategic information to drive new projects and consider changes in legislation or public policies to encourage support for the scientific and technological development. Furthermore, the development of new denture adhesives has a great international market potential that may reflect in an increase in R and D activities and patent applications in this area.
Conclusions
Based on this systematic review and technological monitoring it was possible to obtain a scientific-technological overview of denture adhesives. It was found that health safety requirements and regulamentation issues may represent a favorable scenario for new R and D projects, especially in many underdeveloped countries.
Acknowledgements
The authors thank the financial support from the Brazilian National Council for Scientific and Technological Development (CNPq) (Universal Grant 460588/2014-1).
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
WLOR searched the literature, contributed in data extraction and analysis, and wrote the initial draft of manuscript; SDGO have been involved in drafting the manuscript and revising it critically for important intellectual content; CHR searched the literature, contributed in data extraction and analysis; AFS have been involved in drafting the manuscript and revising it critically; RGL revised the manuscript critically and finished the final version; EP have been involved in drafting the manuscript and revising it critically. All authors read and approved the final manuscript.
6519
References
- Douglass CW, Shih A, Ostry L (2002) Will there be a need for complete dentures in the United States in 2020? J Prosthet Dent 87: 5-8.
- Carlsson GE, Omar R (2010) The future of complete dentures in oral rehabilitation. A critical review. J Oral Rehabil 37: 143-156.
- Psillakis JJ, Wright RF, Grbic JT, Lamster IB (2004) In practice evaluation of a denture adhesive using a gnathometer. J Prosthodont 13: 244-250.
- Bartlett DW, Maggio B, Targett D, Fenlon MR, Thomas J (2013) A preliminary investigation into the use of denture adhesives combined with dietary advice to improve diets in complete denture wearers. J Dent 41: 143-147.
- Figueiral MH, Fonseca PA, Pereira-Leite C, Scully C (2011) The effect of different adhesive materials on retention of maxillary complete dentures. Int J Prosthodont 24: 175-177.
- Polyzois G, Stefaniotis T, Papaparaskevas J, Donta C (2013) Antimicrobial efficacy of denture adhesives on some oral malodor-related microbes. Odontology 101: 103-107.
- Kumar MS, Thombare RU (2011) A Comparative Analysis of the Effect of Various Denture Adhesives Available in Market on the Retentive Ability of the Maxillary Denture: An In Vivo Study. J Indian ProsthodontSoc11:82-88.
- Adisman IK (1989) The use of denture adhesives as an aid to denture treatment. J Prosthet Dent 62: 711-715.
- Hong G, Maeda T, Hamada T (2010) The effect of denture adhesive on bite force until denture dislodgement using a gnathometer. Int Chin J Dent 10:41-45.
- Felton D, Cooper L, Duqum I, Minsley G, Guckes A, Haug S et al. (2011) Evidence-based guidelines for the care and maintenance of complete dentures: a publication of the American College of Prosthodontists. J Prosthodont20 Suppl 1:S1-S12.
- Kalra P, Nadiger R, Shah FK (2012) An investigation into the effect of denture adhesives on incisal bite force of complete denture wearers using pressure transducers - a clinical study. J AdvProsthodont4: 97-102.
- Polyzois G, Partalis C, Lagouvardos P, Polyzois H (2014) Effect of adaptation time on the occlusal force at denture dislodgement with or without denture adhesive. J Prosthet Dent 111: 216-221.
- Agarwal SK, Praveen G, Gupta S, Tandon R, Gupta S (2011) In vitro evaluation of cytotoxicity of denture adhesives. Indian J Dent Res 22: 526-529.
- Koronis S, Pizatos E, Polyzois G, Lagouvardos P (2012) Clinical evaluation of three denture cushion adhesives by complete denture wearers. Gerodontology 29: e161-169.
- Bogucki ZA (2013) Clinical aspects of the use of dental adhesive materials in patients with chronic xerostomia.Gerodontology 30: 162-166.
- Chen F, Wu T, Cheng X (2014) Cytotoxic effects of denture adhesives on primary human oral keratinocytes, fibroblasts and permanent L929 cell lines.Gerodontology 31: 4-10.
- Sampaio-Maia B, Figueiral MH, Sousa-Rodrigues P, Fernandes MH, Scully C (2012) The effect of denture adhesives on Candida albicans growth in vitro. Gerodontology 29: e348-356.
- Grasso JE (2004) Denture adhesives. Dent Clin North Am 48: 721-733, vii.
- Lee C, Cho Y, Seol H, Park Y (2012) A stochastic patent citation analysis approach to assessing future technological impacts. Technological Forecasting and Social Change 79:16-29.
- Nosella S, Petroni G, Salandra R (2007) Technological change and technology monitoring process: Evidence from four Italian case studies. J EngTechnol Manage 25:321-337.
- Barroso W, Quoniam L, Pacheco E (2009) Patents as technological information in Latin America. World Patent Information31:207-215.
- Choi C, Park Y (2009) Monitoring the organic structure of technology based on the patent development paths. Technological Forecasting and Social Change 76: 754–768.
- Cunningham SW, Sanz A (2011) Technology monitoring and analysis: enhancing technological intelligence and competitiveness of small and medium sized enterprises. Tech Monitor:11-9.
- Park H, Kim K, Choi S, Yoon J (2013)A patent intelligence system for strategic technology planning. Expert Systems with Applications40:2373–2390.
- Park H, Yoon J, Kim K (2013) Using function-based patent analysis to identify potential application areas of technology for technology transfer. Expert Systems with Applications 40: 5260-5265.
- Dou H, Leveillé V, Manullang S, Dou Jr JM(2005) Patent analysis for competitive technical intelligence and innovative thinking. Data Science Journal4:209-237.
- Curran C, Leker J(2011) Patent indicators for monitoring convergence – examples from NFF and ICT. Technological Forecasting & Social Change78:256-173.
- Telang MA, Bhutkar SP, Hirwani RR (2013) Analysis of patents on preeclampsia detection and diagnosis: a perspective. Placenta 34: 2-8.
- Chen S, Huang M, Chen D (2012) Identifying and visualizing technology evolution: A case study of smart grid technology. Technological Forecasting & Social Change 79:1099-1110.
- Chen Y, Chen B (2011) Utilizing patent analysis to explore the cooperative competition relationship of the two LED companies: Nichia and Osram. Technological Forecasting & Social Change 78:294-302.
- Hsieh C (2013) Patent value assessment and commercialization strategy. Technological Forecasting & Social Change 80:307-319.
- Thomas VJ, Sharma S, Jain SK (2011) Using patents and publications to assess R&D efficiency in the states of the USA World Patent Information33:4-10.
- Moher D, Liberati A,Tetzlaff J, Altman DG, Group P(2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of clinical epidemiology 62:1006-1112.
- Son C, Suh Y, Jeon J, Park Y (2012) Development of a GTM-based patent map for identifying patent vacuums. Expert Systems with Applications 39:2489–500.
- Uchoa NN, Ferreira RP,Sachetto-Martins G, Mu¨ller AC(2011) Ten years of the genomic era in Brazil: Impacts on technological development assessed by scientific production and patent analysis. World Patent Information 33:150-156.
- Wong C, Goh K (2012) The sustainability of functionality development of science and technology: Papers and patents of emerging economies. Journal of Informetrics6:55-65.
- Duqum I, Powers KA, Cooper L, Felton D (2012) Denture adhesive use in complete dentures: clinical recommendations and review of the literature.Gen Dent 60: 467-477.
- Grasso JE (1996) Dentureadhesives: changing attitudes.J Am Dent Assoc 127: 90-96.
- Gupta R, Luthra RP (2012) Denture Adhesives: A Review. Indian Journal of Dental Sciences 4:85-87.
- Tezvergil-Mutluay A, Ca rvalho RM, Pashley DH (2010) Hyperzincemia from ingestion of denture adhesives. J Prosthet Dent 103: 380-383.
- Shay K (1991) Denture adhesives. Choosing the right powders and pastes.J Am Dent Assoc 122: 70-76.
- Polyzois GL, de Baat C (2012) Attitudes and usage of denture adhesives by complete denture wearers: a survey in Greece and the Netherlands.Gerodontology 29: e807-814.
- Da Rosa WLO, Silva AF, Oliveira AS, Lund RG, Leites ACBR, et al. (2014) Technological monitoring of dentin desensitizing agents. RFO Passo Fundo 19:107-114.
- Mazzolenia R(2005) University patents, R&D competition, and social welfare. Economics of Innovation and New Technology. 14:499-515.
- Sampat BN(2006) Patenting and US academic research in the 20th century: the world before and after the Bayh-Dole. Research Policy35:772-789.
- Ernst H (2003) Patent information for strategic technology management. World Patent Information25:233-242.










