Introduction
Plerixafor (AMD3100, Mozobil) is a new targeted drug that specifically binds and blocks the CXCR4 chemokine receptor. Primarily, it was designed for therapy of HIV infection, because CXCR4 is used by T-lymphotropic HIV strains to enter the cells. The first clinical trials in AIDS were not successful, however led to the identification of an unexpected side effect: increase in the white blood cell count. When this phenomenon was further investigated, it was found that administration of plerixafor increases the number of hematopoietic stem cells (HSC) circulating in the peripheral blood of patients (PBSCs). This in turn, led to the hypothesis that plerixafor may find its application in HSC mobilization for the purpose of peripheral blood stem cell transplantation (PBSCT) [1].
At present, the most common stem cell mobilization strategy is based on administration of granulocyte-colony stimulating factor (G-CSF) alone or in combination with chemotherapy. The action of G-CSF is believed to be based on activation of cells of neutrophil lineage. These cells secrete proteolytic enzymes that cleave adhesion molecules attaching HSC to bone marrow stroma [2]. Impairment of interaction between SDF-1 chemokine, which is expressed by bone marrow stromal cells and CXCR4 receptor at the surface of HSCs seems to be one of the most important effects of G-CSF. As a result, a proportion of HSCs leaves their bone marrow niche and enters the circulation. Administration of chemotherapy prior to G-CSF provides additional trigger for HSC release from the niche and significantly enhances HSC mobilization [2].
Unfortunately, the current strategies used for hematopoietic stem cell mobilization fail in significant number of patients. About 5-30% of patients do not succeed to collect > 2.0 x 106 CD34+ cells/kg b.w. during first mobilization attempt and this number is usually required for successful stem cell engraftment [2,3]. Unfortunately, the remobilization in these patients who failed previous mobilization seems to be related with low efficiency. In a series of patients reported by Pusic et al. (2008), only 23% of remobilized patients achieved > 2 x 106 CD34+ cells/kg in second collection and 29.7% failed to pool sufficient number of stem cells from both collections.
Importantly, several clinical studies revealed that plerixafor, when used in combination with G-CSF may be very effective even in patients who fail previous mobilization attempt [4,5,6,7].In Europe, plerixafor was used since 2007 within the Compassionate Use Protocol. Calandra et al. (2008) reported the first series of patients remobilized with plerixafor in combination with G-CSF in Europe and the rates of successful CD34+ cell collection were satisfactory: 60-95% depending on the primary disease diagnosis.
The major mechanism of plerixafor action is believed to be based on direct blocking of CXCR4 receptor expressed by HSCs and progenitors. In such situation, interaction between SDF-1 and HSCs is no longer possible and the balance between factors keeping HSCs in BM microenvironment and their recirculation is being disturbed. However, the exact mechanisms of mobilization with plerixafor are largely unknown, yet. Recently, it was suggested that plerixafor binding to CXCR4 receptor stimulates BM neutrophils which activate complement cascade [8]. In turn, complement activation may be essential for efficient stem cell mobilization.
The current publication summarizes our single-institution experience on the use of plerixafor for HSC mobilization in 16 patients suffering from multiple myeloma or lymphoma. All of them either failed previous mobilization attempt or were predicted poor mobilizers based on low HSC concentration after conventional treatment. Importantly, we report for the first time high nucleated cell count after plerixafor-based mobilization which, in our setting, resulted in high volumes of stem cell product.
Patients and Methods
The group of 16 patients was heterogenous (Table 1). Ten of them suffered from multiple myeloma, three of Hodgkin’s lymphoma (HL), one of mantle cell lymphoma (MCL) and two of diffuse large B cell lymphomas (DLBCL). The median age of patients was 59 (range, 19-71). Seven of them were females and nine were males. Only 3 patients were in complete remission, while the others had active disease. The majority of patients have already been heavily pretreated and received median of 9 (range, 4-27) previous chemotherapy courses. Chemotherapy regimens are listed in Table 1. Four patients were also treated with radiotherapy before and five patients were mobilized after previous autologous PBSCT (autoPBSCT). Thirteen patients failed at least one previous chemotherapy-based stem cell mobilization attempt.
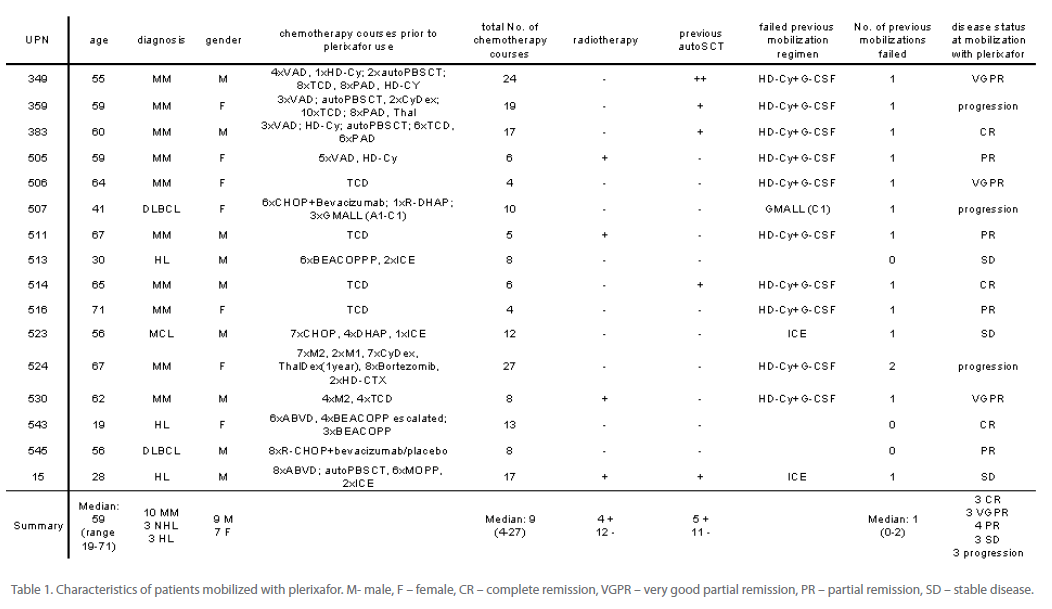
Table 1: Characteristics of patients mobilized with plerixafor. M- male, F – female, CR – complete remission, VGPR – very good partial remission, PR – partial remission, SD – stable disease.
Plerixafor (Mozobil) was generously provided by the manufacturer (Genzyme) within the Compassionate Use Protocol. The drug was administered according to the manufacturer’s recommendations (Figure. 1) with some modifications. In brief, beginning from day one, patients have received G-CSF at a dose of 2 x 5 μg/ kg body weight. On day four, patients received first s.c. injection of plerixafor at a dose of 240 μg/kg b.w. at 11 p.m. The following day, the complete blood count (CBC) was recorded and peripheral blood CD34+ cell concentration was assessed by flow cytometry. The absolute No. of CD34+ cells/μL was assessed according to formula: WBC count/μL x proportion of CD34+ cells in nucleated cell fraction. When it reached satisfactory level (> 15 CD34+ cells/μL), leukaphereses started (at about 10 a.m.).
In patients UPN 513, 543 and 545, plerixafor was added to chemotherapy- based mobilization regimen. They received standard chemotherapy and G-CSF at a dose of 2 x 5 μg/kg b.w. since day five. Patients UPN 543 and 545 received high-dose (4000 mg/m2) cyclophosfamide, while patient UPN 513 was treated with ICE regimen (etoposide 100 mg/m2, day 1-3; ifosfamide 5000 mg/m2, day 2; carboplatin 800 mg, day 2). The decision when to co-administer plerixafor was made based on observation of low (< 15/μL) CD34+ cell count in the peripheral blood at optimal time for stem cell collection (day ten to fourteen after chemotherapy). At this time, the blood leukocytosis was > 3.5 G/L and raising without satisfactory rise in CD34+ cell No./μL. Administration of plerixafor at this time aimed in boosting stem cell release to the peripheral blood.
For the purpose of cell collections COBE Spectra cell separator was used, equipped with version 6.1 software. Manual MNC procedure was applied with the aim of processing of 2 times the Total Blood Volume. Treated patient’s blood volume, procedure length and volume of immediate product for each separation are listed in Table 2. We used the routine protocol for leukapheresis that was elaborated in our institution for patients mobilized after chemotherapy-based regimen and we did not modify separation factor (SF) which was set by an authorized service. Cell populations within leukapheresis product were assessed by standard blood morphology and flow cytometry. For CD34+ cell enumeration the double cytometric platform was used (as described above). The pre-thaw minimal residual disease was assessed also by flow cytometry. For this purpose, in multiple myeloma patients the proportion of CD138+ cells (plasma cells) was assessed. The leukapheresis product was frozen at density of < 50 x 106 nucleated cells (NCs)/mL in final solution containing 5% dimethylsulfoxide (DMSO). The minimum target of collections was > 2 x 106 CD34+ cells/kg b.w. If it was not reached after the first stem cell collection, administration of plerixafor, G-CSF and leukaphereses were repeated as above until target No. of cells was collected. The optimum target of collections was > 4 x 106 CD34+ cells/kg b.w. that allows for two autoPBSCT. Therefore, in patients who already collected minimum cell target, leukaphereses were continued, to the maximum number of 3. In patients with low peripheral blood CD34+ cell count and low yield from first leukapheresis, the decision about next leukapheresis was made based on peripheral blood CD34+ cell count following second plerixafor administration. If it fell below minimum threshold, leukaphereses were omitted.
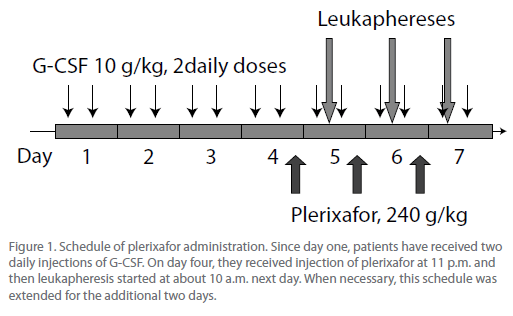
Figure 1: Schedule of plerixafor administration. Since day one, patients have received two daily injections of G-CSF. On day four, they received injection of plerixafor at 11 p.m. and then leukapheresis started at about 10 a.m. next day. When necessary, this schedule was extended for the additional two days.
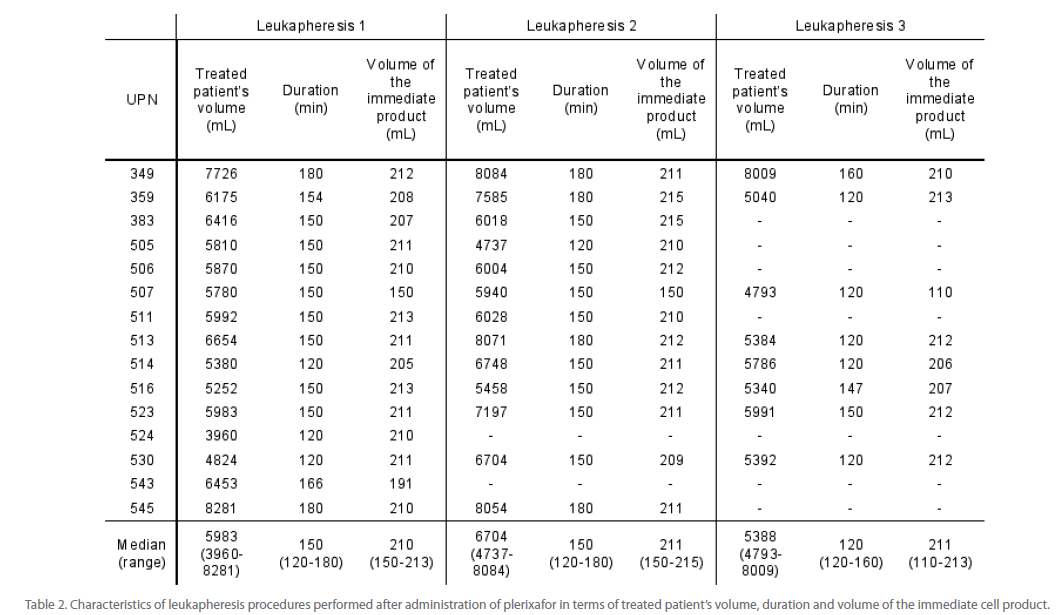
Table 2: Characteristics of leukapheresis procedures performed after administration of plerixafor in terms of treated patient’s volume, duration and volume of the immediate cell product.
Results
Mobilization with G-CSF and plerixafor was associated with satisfactory release of CD34+ cells to the peripheral blood as measured 8 hours after plerixafor administration. The median of 23 CD34+ cells/μL was achieved and ranged between 11 and 62 cells/μL (Table 3). The lower limit of 15 CD34+ cells/μL which was required to start leukapheresis, was achieved in 13 out of 16 patients after first, and in next two patients after second administration of plerixafor. In patient UPN 15, the No. of CD34+ cells in peripheral blood was only 13/μL on the first day after plerixafor administration and fell to 5 CD34+ cells/μL after second plerixafor administration. Therefore, this patient did not fulfill criteria to start cell collection. In all remaining patients (15/16), leukaphereses were performed.
However, CD34+ cell release from bone marrow niche was associated with mobilization of neutrophils which contributed to the unexpectedly high peripheral blood leukocytosis. While on the day of plerixafor administration the median WBC count (G/L) was 28 (range, 6.9-53.8), it rose to 38.4 (range, 11-72) after first, 48.5 (16.8-88.7) after second and 59.2 (21-121.6) after third plerixafor administration (Figure 2). As a consequence, the frequency of CD34+ cells among WBC was low - median 0.067% after first plerixafor administration, range 0.03-0.215 (Table 3). Patients UPN 383 and 505 did not release required No. of CD34+ cells after first plerixafor administration, but achieved this goal after second dose of plerixafor and went into leukaphereses. In total, median of 3 leukaphereses were performed (range 0-3) and the median collected CD34+ cell No. was 2.8 x 106 CD34+ cells/kg b.w. (range, 0.57 - 4.5 x 106 CD34+ cells/kg b.w. (Table 4). The minimum target of > 2.0 x 106 CD34+ cells/ kg b.w. was collected in 12/16 patients (75%) in median of 2 leukaphereses (range, 1-3). The optimum target of collections (> 4.0 x 106 CD34+ cells/kg b.w.) was obtained in patients UPN 506 and 507 after second and third leukapheresis, adequately. In patient UPN 543 who collected 2.3 x 106 CD34+ cells/kg in first leukapheresis, further collections were not performed based on clinical decisions. Also in patient UPN 545 leukaphereses were stopped after collection of 3.4 x 106 CD34+ cells/kg because it reached the optimum of 4.0 x 106 CD34+ cells/kg when pulled with previously collected and stored material.
The proportion of CD34+ cells in stem cell product was low (median 0.25%; range, 0.09-0.81) due to high No. of nucleated cells (NCs) in preparations (median 9.3 x 108 NCs/kg b.w.; range 6.15-24.05) (Table 5). Based on institutional cryopreservation procedures, the volume of frozen cell preparations was high (median 1260 mL) and ranged between 500 and 2050 mL. In MM patients the median proportion of CD138+ cells in stem cell product was 0.03%, but ranged between 0.01% and 1.15%. This translated into median of 0.28 x 106 CD138+ cells/kg b.w. (range, 0.007-4.1). In patient UPN 359 it was almost four times more than the No. of CD34+ cells collected (10.67 vs. 2.6 x 106 cells/kg b.w.). The median No. of CFU-GEMM + CFU-GM as well as BFU-E colonies are listed in Table 5.
To date, eight out of sixteen patients underwent autoSCT. In all of them hematopoietic stem cells engrafted and the median time to neutrophil recovery > 0.5 G/L was 12.5 days (range, 11- 14) and to platelet recovery >20G/L was 14 days (range, 10-25) (Table 6). We did not observe late graft failure in these patients.
Importantly, we did not observe any side effects of HSC mobilization with plerixafor.
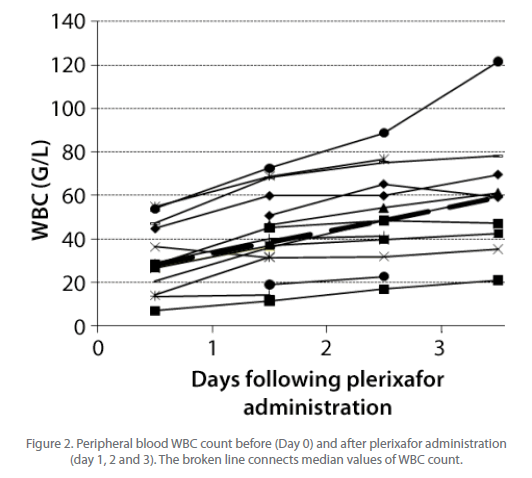
Figure 2: Peripheral blood WBC count before (Day 0) and after plerixafor administration (day 1, 2 and 3). The broken line connects median values of WBC count.
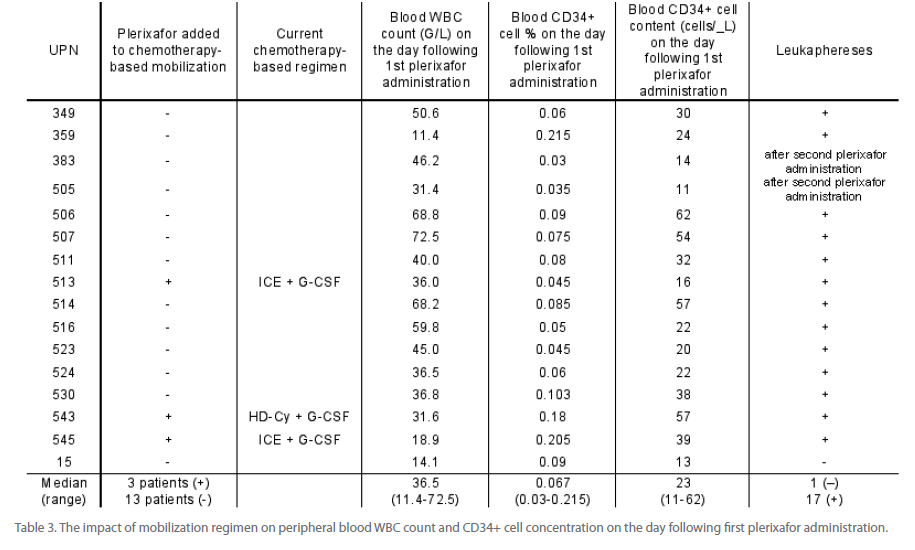
Table 3: The impact of mobilization regimen on peripheral blood WBC count and CD34+ cell concentration on the day following first plerixafor administration.
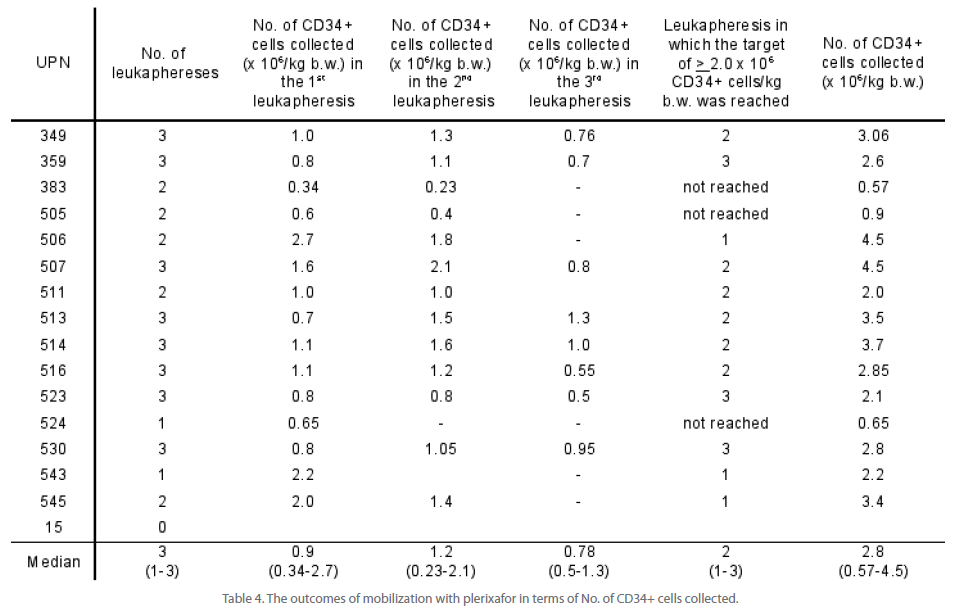
Table 4: The outcomes of mobilization with plerixafor in terms of No. of CD34+ cells collected.

Table 5: Characterization of collected stem cell product.
Discussion
Plerixafor has been recently (2009) approved by the European Medicines Agency (EMEA) “to enhance mobilization of HSCs to peripheral blood for collection and subsequent autologous transplantation in patients with lymphoma and multiple myeloma whose cells mobilize poorly” [9]. This was based on the results of two key multicenter, randomized, double-blind, placebo controlled studies of plerixafor plus G-CSF versus placebo plus G-CSF for autologous stem cell mobilization in patients with non-Hodgkin’s lymphoma and myeloma [6,10]. In non- Hodgkin’s lymphoma patients, the primary goal of 5.0 x 106 CD34+ cells/kg in four or fewer days of apheresis was achieved in 59% of patients in the plerixafor arm compared with 20% in the placebo arm (p<0.0001). In multiple myeloma study, a higher end-point of > 6.0 x 106 CD34+ cells/kg in two or less days of apheresis was used, and 72% of patients treated with plerixafor and G-CSF achieved this goal compared with 34% of patients treated with G-CSF and placebo (p<0.0001). These studies clearly showed that treatment with plerixafor is superior than placebo and the results of such mobilization are satisfactory. However, these studies have been performed in patients who did not fail previous mobilization attempt. Moreover, multiple exclusion criteria were applied, such as previous thalidomide or lenalidomide treatment, radiotherapy or autoPBSCT, which may suggest that chosen patients had higher chance of successful mobilization than patients usually seen in the clinic. Therefore, it is of high importance to document the real efficiency of plerixafor-based mobilization regimen in patients who are predicted or proven poor mobilizers. This is the first report of mobilization with plerixafor in combination with G-CSF within the Compassionate Use Programme in Poland.
Our results confirm the high mobilizing efficiency of plerixafor. Satisfactory CD34+ cell number in the peripheral blood of > 15/ μL was observed in 13/16 patients after first application of plerixafor which allowed to start leukapheresis. Patients UPN 383 and 505 who achieved the minimum limit of 15 CD34+ cells/ μL after second administration of plerixafor only, failed to collect required No. of cells. The median CD34+ cell No/μL in our patients (23 cells/μL) was lower than in majority of published prospective clinical studies. However, this most likely resulted from patient’s selection. Stewart et al. [11] observed median of 75 CD34+ cells/μL after first administration of plerixafor. However, in that case the No. of circulating CD34+ cells before plerixafor (24 cells/μL) already exceeded numbers observed by us after drug administration that suggests the impact of different patient’s population. The centre-specific method of CD34+ cell enumeration may also influence differences between centers. Moreover, the majority of studies did not reveal the No. of circulating CD34+ cells, but rather fold increase compared with day prior to plerixafor administration. The first and largest retrospective study by Calandra et al. [7] also does not reveal these numbers.
Similarly, these studies did not reveal WBC count during mobilization with plerixafor and G-CSF, while we have observed very high leukocytosis, secondary to the increase in the neutrophil count. It was observed already in healthy volunteers that single injection of plerixafor without G-CSF may lead to increase in WBC count of up to 19 G/L [12]. However in case of mobilization with plerixafor in combination with G-CSF, the leukocytosis most likely results from administration of growth factor, as observed before plerixafor administration. On days before and following each plerixafor administration, the median rise in leukocytosis was about 10 G/L per day, however G-CSF was also administered at this time. High leukocytosis could be also related with administration of G-CSF in divided daily dose (2 x 5 μg/kg), instead of single daily dose (10 μg/kg) used by other investigators. However, this explanation is unlikely as study by Kroger et al. did not reveal any difference in peripheral blood WBC count between once-daily or twice-daily schedule [13]. On the other hand, such dosing regimen was shown to be superior in terms of CD34+ cells mobilization [13].
We managed to collect minimum No. of > 2.0 x 106 CD34+/kg b.w. in 75% of patients. The success rate was higher than reported by Calandra et al. [7] (66%) and this may result from inclusion in our study of patients who were predicted poor mobilizers. In our report, all those patients who received plerixafor as rescue of their primary chemotherapy-based regimen, collected required No. of cells. This suggests the potential future escalation of such strategy. Importantly, the median (or mean) No. of CD34+ cells/kg b.w. collected by our patients was lower. In a series of patients reported by Calandra, the mean total CD34+ cell yield was 3.5 x 106/kg b.w. compared with our results of 2.6 x 106 CD34+ cells/kg b.w. This may be affected by cell collection procedure and related with observation of high leukocytosis in our patients.
The association between high WBC count and poor CD34+ cell yields was already reported by Burgstaler et al. [14,15] and this could affect the relatively low number of CD34+ cells collected. Moreover, the high leukocytosis led to the low frequency of CD34+ cells within WBC fraction and therefore, in order to collect sufficient No. of CD34+ cells, we had to collect abundant No. of NCs. Importantly, our leukapheresis system was adapted to cell collections in patients mobilized with chemotherapybased regimens who usually have relatively low WBC count. In case of high leukocytosis, the contamination of material with neutrophils was significant and this resulted in high NCs count in stem cell product. In turn, the observed high NCs count led to high volume of frozen stem cell product. The procedure of cell freezing requires certain NCs concentration in order to maintain their viability. In our institution this is not more than 50 x 106 NCs/mL of freezing medium. This resulted in median volume of 1260 mL of frozen stem cell product, which is very high. This observation has not been reported previously.
High leukocytosis and resulting low collection efficiency, large number of collected NCs and resulting large volumes of frozen stem cell product, seem to be a clear disadvantage of mobilization with plerixafor in combination with G-CSF. Therefore, it will be very important to elaborate certain strategies to solve them. The high leukocytosis could be avoided by addition of plerixafor to chemotherapy-based mobilization regimens at the time when leukocytosis is low but raising. In our three patients, where plerixafor was used as rescue of previously applied regimens, it was administered when leukocytosis was already high. High leukocytosis may also require modification of leukapheresis protocols. It was suggested that, in this setting, slowing the flow rate or increasing the separation factor during the collection may enhance CD34+ cell collection efficiency and decrease the granulocyte content of the product [15]. In the situation when the No. of collected NCs containing required CD34+ cell fraction is already high, one could suggest cell freezing at higher density than 50 x 106 NCs/mL. However, based on our experience, this may result in disintegration of necrotic granulocytes after cell thawing resulting in cell clumping by released DNA. Therefore, the possible complications of such approach seem to support cell freezing at already established density. Being aware of side effects resulting from single infusion of large volumes of DMSO-containing, ice cold fluid during transplantation, we usually divided the stem cell products into two to three infusions carried on consecutive days. Such practice did not produce side effects besides transient nausea and vomiting.
Ten patients mobilized with plerixafor already underwent autologous SCT. Similarly as in other studies, we did not observe altered kinetics of platelet and neutrophil recovery after transplantion of plerixafor mobilized cellular product. We also did not observe long-term graft failure, however the time of observation was limited.
In summary, plerixafor has been shown to be efficient and useful for stem cell mobilization in myeloma and lymphoma patients who failed previous mobilization attempt. However, mobilization with plerixafor in combination with G-CSF is resulting in high leukocytosis, subsequent low frequency of CD34+ cells and high volume of obtained stem cell product.
Abbreviations:
G-CSF – granulocyte-colony stimulating factor
NCs – nucleated cells
HSC – hematopoietic stem cell
PBSC – peripheral blood stem cell
PBSCT – peripheral blood stem cell transplantation
WBC – white blood cells
CFU-GEMM – colony-forming unit granulocyte-erythrocyte –megakaryocyte-macrophage
BFU-E – burst forming unit erythrocyte
SCT – stem cell transplantation.
Conflict of Interest
Grzegorz W. Basak received reimbursement for attending symposia and honoraria from Genzyme. The remaining authors declare no conflicts of interest.
Funding
Plerixafor was provided by the Genzyme Corp. free of charge within Compassionate Use Program (CUP).
Acknowledgements
The authors would like to thank Mrs. Malgorzata Krol, Mrs. Malgorzata Zdzieblowska and Mrs. Ewa Lowkiewicz for their excellent help with cell separation and processing.
2595
References
- De Clercq E (2009) The AMD3100 story: the path to the discovery of a stem cell mobilizer (Mozobil). Biochem Pharmacol 77: 1655-1664.
- Bensinger W, DiPersio JF, McCarty JM (2009) Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant 43: 181-195.
- Villalon L, Odriozola J, Larana JG, Zamora C, Perez de Oteyza J, et al. (2000) Autologous peripheral blood progenitor cell transplantation with <2 x 10(6) CD34(+)/kg: an analysis of variables concerning mobilisation and engraftment. Hematol J 1: 374-381.
- Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, et al. (2005) The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 106: 1867-1874.
- Micallef IN, Stiff PJ, DiPersio JF, Maziarz RT, McCarty JM, et al. (2009) Successful stem cell remobilization using plerixafor (mozobil) plus granulocyte colony-stimulating factor in patients with non-hodgkin lymphoma: results from the plerixafor NHL phase 3 study rescue protocol. Biol Blood Marrow Transplant 15: 1578-1586.
- DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, et al. (2009) Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non- Hodgkin’s lymphoma. J Clin Oncol 27: 4767-4773.
- Calandra G, McCarty J, McGuirk J, Tricot G, Crocker SA, et al. (2008) AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non- Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant 41: 331-338.
- Lee HM, Wysoczynski M, Liu R, Shin DM, Kucia M, et al. (2010) Mobilization studies in complement-deficient mice reveal that optimal AMD3100 mobilization of hematopoietic stem cells depends on complement cascade activation by AMD3100-stimulated granulocytes. Leukemia 24: 573-582.
- EMEA (2009) Mozobil. In: Agency EM, editor. EPARs for authorised medicinal products for human use -.
- . DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, et al. (2009) Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 113: 5720-5726.
- Stewart DA, Smith C, MacFarland R, Calandra G (2009) Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma. Biol Blood Marrow Transplant 15: 39-46.
- Hubel K, Liles WC, Broxmeyer HE, Rodger E, Wood B, et al. (2004) Leukocytosis and Mobilization of CD34+ Hematopoietic Progenitor Cells by AMD3100, a CXCR4 Antagonist. Support Cancer Ther 1: 165-172.
- Kroger N, Sonnenberg S, Cortes-Dericks L, Freiberger P, Mollnau H, et al. (2004) Kinetics of G-CSF and CD34+ cell mobilization after once or twice daily stimulation with rHu granulocyte-stimulating factor (lenograstim) in healthy volunteers: an intraindividual crossover study. Transfusion 44: 104-110.
- Burgstaler EA PA (2002) The negative effect of high peripheral white blood cell count on CD34+ cell recovery. Journal of Clinical Apheresis 17: 148.
- Burgstaler EA PA, Winters JL (2003) Effects of high whole blood flow rates and high peripheral blood cell counts on CD34+ cell yield and cross-cellular contamination. Cytotherapy 5: 446.












