Nickson J Kawung1*, Remy EP Mangindaan1, Rizald M Rompas1, Ekowaty Chasanah2, Megy Kapoyos1, Boby Abdjul1, Hedi I Januar2, Dewi Fajarningsih2 and Adolfina Sumagando3
1Faculty of Fisheries and Marine Sciences, Sam Ratulangi University, Indonesia
2Indonesian Research and Development Center for Marine and Fisheries Product Processing and Biotechnology, Jl. KS Tubun Petamburan VI, Jakarta 10260, Indonesia
3Faculty of Mathematics and Natural Science, Christian University, Indonesia
*Corresponding Author:
Nickson J Kawung
Faculty of Fisheries and Marine Sciences
Sam Ratulangi University, Indonesia
Tel: 6285395373630
E-mail: kawungnickson@yahoo.com
Received date: June 06, 2017; Accepted date: July 12, 2017; Published date: July 14, 2017
Citation: Kawung NJ, Mangindaan REP, Rompas RM, Chasanah E, Kapoyos M (2017) Cytotoxic Anticancer from New Compound Unsrat-sinularine of Softcoral Sinularia Sp. from Bunaken Island, Manado, Indonesia. Int J Drug Dev & Res 9:01-04
Keywords
Sinularia sp; Elucidation; Anticancer; Fraction
Introduction
Indonesia is famous for its great biodiversity characterized with high levels of both from phenotype and genotype point of view. Romps stated that 17% of marine biota can be found in Indonesia and they have high biodiversity [1]. This definitely affects the possibility of having bioactive compounds from marine biota. Exploitation and exploration of active compounds from each biota have become interesting topics because these compounds are very important in pharmacy, among them bioactive compound from soft coral.
Buna ken Island is one of national parks in Manado Indonesia. This island has a high marine biodiversity. There have been many research on bioactive compounds from marine biota taken from Buna ken Island waters but most of them were on Sponge and Ascidian. Research on soft coral is still few and limited only on crude exact test, while finding the active structure is also still limited. Generally, compounds produced by organism including soft coral which have biological active substances are categorized as secondary metabolites. Sinularia is one soft coral which has bioactive compound that can be used in pharmaceutical industry. The content and chemical structure of bioactive compound from soft coral are affected by ecological condition where the coral live. Besides, there is also a possibility that the components of the compound are different from other compounds from Sinularia sp. from other locations. According to Januar et al., there is a variation of cembranoids in soft coral Sarchophyton sp. and Nephthea sp. from different environmation [2]. Pawlik stated that different ecological interaction can cause different composition of compounds of natural products secreted [3]. Based on that, research to identify compound structure of soft coral Sinularia sp. from Bunaken Island in North Sulawesi is important to be done to determine type of active compound in there. Beside that, the research is also aimed to obtain information about bioactivity and model of the structure of the compound in order to use it as a comparison for research on active compounds taken from soft coral Sinularia sp. or other soft coral from different ecological conditions.
Characterization of pure compound from natural product can be done with purification method followed by spectroscopically identification. Isolation and structural elucidation of chemical structure are generally conducted by using a combination of instruments for chemical analysis such as Column Chromatography C18 to separate minerals of the extract and High Pressure Liquid Chromatography preparative for the fractionation of the active compounds, and LC-MS as low resolution mass detector at the preliminary stage of identification, while FT-MS as high resolution mass detector to obtain molecular formula of the targeted compound. Spectrometer NMR is used to identify the number of proton in the compound.
The proportion of the compounds can be obtained by using Database Chemispider and marinLit 2014. This Database contains updated chemical structures from marine organisms. Besides, the use of the database is also important to avoid identification of compounds which thought to be new compounds.
Materials and Methods
Soft coral Sinularia sp. was taken from the waters around Buna ken Island in North Sulawesi Indonesia on February 2014. The sample was obtained by a diver with scuba diving apparatus from the depth of 5 to 10 meter. After being obtained, the sample was weighed and put into a plastic bottle containing methanol and immediately macerated with 200 ml of methanol. After 48 hours, the sample was sieved and macerated again with a 20 ml methanol. The procedure was repeated twice. The solution was then evaporated at 30°C until it become concentrated. In order to remove all the solvent, the extracted solution was then dry-frozen with freeze dryer until it powdered. The powdered sample was dissolved with the mixture of methanol:dichloroethane (1:1) and elucidated through column chromatography C18. The solution was then evaporated at 30°C until it concentrated and was dried with freeze dryer. The powder was again dissolved with Methanol HPLC to 2 ml and fractionated with HPLC Preparative (Shimadzu).
Characterization of Bioactive Compounds
Active fraction from the previous compound was then analyzed for its purity by using 1H-NMR (Jeol ECS 400 MHz). Pure isolates were then characterized structurally by the pattern of the mass spectrometer (Shimadzu LCMS IT-TOF mass spectrometer) and ID and 2D-NMR, the spectra are referenced to the 1H resonance and 13C deuterated solvents. Further cytotoxicity analysis of each isolate was done by Zachary methods to determine the value of IC50 value of each active isolates found [4-18].
Structural Elucidation of Bioactive Isolate of Anti- Cancer
Elucidation of bioactive isolates was conducted using data from the chemical sliding of each proton and carbon compound on NMR instrumentation (JEOL ECS NMR 400 MHz), molecular weight data and UV-VIS absorption on LC MS instrumentation (LCMS DAD ITTOF Shimadzu Hybrid Ion Trap Time of Flight).
Molecular Weight Analysis by LC-MS and TMS Method
Active isolates were dissolved in 100 μL of methanol, and then injected into the stationary phase of LCMS system on return silica of C18 (Prep ODS Shimadzu 250 mm × 20 mm), eluted at 15 mL/min by using a gradient of 10% acetonitrile in water to 100% setonitril for 20 minutes and isocratic 100% acetonitrile for 10 minutes (total method on mobile phase was 30 minutes), by DAD detector (Diode Array detector) at a wavelength of 200-800 nm, and accurate mass detector.
Data Measurement of Chemical Sliding on Proton and Carbon Active Compound
The powdered sample was dissolved with the mixture of methanol:dichloroethane (1:1) and elucidated through column chromatografi C18. The solution was then evaporated at 30°C until it concentrated and was dried with freeze dryer. The powder was again dissolved with Methanol HPLC to 2 ml and fractionated with HPLC Preparative (Shimadzu). The LC-MS analysis was done by injecting 10 μl of the solution to system instrument shimadzu 2010A kolom Phenomenex Luna RP-C18 (150 × 2 mm) by using fase gerak gradient dari 10% acetonitrile-air for 40 minutes. FT-MS analysis was done by injecting a 100 μg/ml of sample to broker Bio Apex 47e Fourier transform Ion Cyclotron Resonance Mass. The elucidation of structure of active compound was determined through 1H-NMR, 13C-NMR with proton interpretation program, COSY, and HMQC. Chemical shift value, was in parts per million (ppm) using tetrametilsilana (TMS) as a standard. The solvents that are being used are chloroform and water NMR.
Determination of Spectroscopic Analysis Data
Determination process begins with the identification of active compounds using overall data of molecular weight, a combination of LC-MS and FT-MS with and NMR data on the electronic database MarineLit 2014. If the corresponding compounds in database are not found, elucidation of active compounds structure will be deductively done manually based on the overall pattern of obtained spectroscopic data (Jenie et al.; Kosela).
Quantitative Test of Anti-Cancer Compound
This test aims to see the IC50 of each test compounds to cancer cells. In this phase, a series making of concentrations was done on compound of four fractions selected as follows. Sample of extract powder was weighed and then dissolved in DMSO. DMSO volume was adjusted to the sample weight and the concentration of the desired stock (100.000 mg/l). The test solution was diluted to 10.000 mg/l and further diluted to 1000 mg/l. The 1000 mg/l test solution was diluted in a series of dose desired by following the above formula and dissolved in culture media. The series of dose used was 30 mg/ml, 15 mg/ml, 7.5 mg/ml, 3.75 ppm, 1.875 mg/l, 0.937 mg/l and 0.468 mg/l. Determination of the dose was taken from the dose of Doxorubin drugs 30 ppm chemotherapy. Test solution was added to the culture medium to a volume of 1000 μL.
IC50 Determination
For cytotoxic test, cancer cell HeLa (cervical cancer) and breast cancer cells (MCF-7 and T47D) were used as test cells. The cells were cultured in complete media RPMI (Roswell Park Memorial Institute) containing fetal bovine serum of 10% in CO2 incubators at a temperature of 37°C using MTT test method {3- (4,5-dimethylthiazol- 20yl) 2-5-diphenyltetrazolium bromide}. The method follows Freshney and Ebada et al. as modified by Nursid M for the whole cytotoxic test, in which the cell count of 10.000 cells/well was used [4]. Absorbance measurement of each well was done by microplate reader at a wavelength of 570 and 690 nm. The percentage of cell death was calculated based on the formula [(xD-xA)-(xb-xc)/(xD-xc)] × 100%. Value of Lethal Concentration (LC50) and Inhibitor Concentration 50 (IC50) was determined using profit analysis with the help of Minitab 16.0 statistical program.
Determination of the Structure of Active Compound
The determination of molecule structure was done to the data of accurate mass from FT-MS result by using Elemental Composition Calculator software (Antolasic). The result of molecule structure from the program was then compared to molecule structure in electronic database electronic of MarinLit 2008 database of several structures from marine biota.
Results
The result of biological anti-cancer test of two new compound using MTT method shows that anti-tumor bioactivity is specific for each cancer cell and profit analysis obtains the value of inhibitor concentration (IC50) is the HeLa 0.64 mg/l, T47D 12.1 mg/l and MCF-7 1.2 mg/l. According to Meyer et al., a substance is considered having potential cytotoxic activity if it has IC50 value at the concentration of <1000 ppm for extract and at concentration ≤ 30 ppm for a compound [5]. Based on the data above, isolated bioactive compound of soft coral sinularia sp from Bunaken Island Indonesia is very toxic to the tested compound. Nursid stated that the smaller the value of IC50 the more toxic is the compound, the opposite, the bigger the value of IC50 the less toxic is the compound [4]. The result of the research by Ching et al., on the extract of sinularia sp from Taiwan shows a low cytotoxic activity toward HeLa Cell, Hep-2 and MCF-7 [6]. Pharmacological activity and compound structure of each biota are frequently affected by the ecological condition where the biota lives. Therefore, in this research there are different pharmacological activities and chemical structure of soft coral sinularia sp from Bunaken Island, Indonesia
The calculation of cancer cell mortality to new compound is the MCF-78, 84%, T47D cell 40.59% and HeLa Cell is 90.33%. HeLa Cell is a kind of line cell that is often used in laboratory for bioactive compound test of anticancer compound studies.
MCF-7 cells are one of the breast cancer cell model which is commonly used in research of the anticancer compound tests. The cells were taken from the breast tissue of a 69-year old woman [7,8] suggested that the cells are chemotherapy-resistant cells. The cells cannot express caspase-3 [9-11] and can only express estrogen alpha receptor so that it resistant toward doxorubicin [9]. T47D cells are also breast cancer cell which were taken from tumor doctor tissue of a 54 year woman. This is an epitel cell and can express the p53 mutated protein. Mutation of the cells occurs at the residual protein 194 (Zinc-binding domain) which can caused the p53 cannot be responded by part of the element in DNA and at the end this p53 lost its ability to regulate cell cycle [12]. HeLa cells (Hela Cell Line) was taken from elite of cervix cancer cells from a patient with cervix cancer named Henrietta Lacks [13]. These cells have the ability to divide without limit. New strains of HeLa cells have been developed in many culture from the same parentage. This cell has been through some transformation because of viral infection of human papillomavirus 18 (HPV 18) [13].
Analysis with NMR, LC-MS and Structure Elucidation
The result structure analysis of the new compound is shown in Figure 1. The new compound has a Carbon (C20), Hydrogen (H28) and Oxygen (O5); molecular formula of C20H28O5 and molecular weight (MW) of 348,19 and OH functional group at Carbon number 6. The spectra analysis NMR (δC dan δH) in the CDCL3 are shown in Table 1.
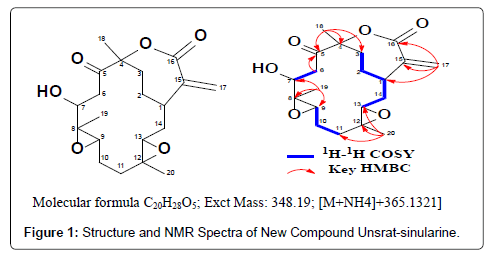
Figure 1: Structure and NMR Spectra of New Compound Unsrat-sinularine.
| No |
δC |
δH |
Mult.(J in Hz) |
COSY |
HMBC |
| 1 |
35.1 CH |
2.31 |
m |
2,14b |
|
| 2 |
30.7 CH2 |
2.49 |
m |
1,3a |
|
| 3 |
33.4CH2 |
1. 96 |
m |
2 |
|
| 4 |
91.4C |
|
|
|
|
| 5 |
208.5C |
|
|
|
|
| 6 |
40 CH2 |
2.68 |
m |
7 |
|
| 7 |
|
2.98 |
m |
7 |
|
| 8 |
71.7 CH |
4.55 |
brd (8.2) |
6a,6b |
|
| 9 |
62.4C |
|
|
|
|
| 10 |
55.0CH |
2.90 |
m |
10a,10b |
|
| 11 |
22.1 CH2 |
1.59 |
m |
9 |
|
| 12 |
|
1.90 |
m |
9, 11a,11b |
|
| 13 |
34.8CH2 |
2.35 |
m |
10b |
|
| 14 |
|
2.40 |
m |
10b |
|
| 15 |
59.8C |
|
|
|
|
| 16 |
62.9CH |
3.01 |
brd (11.1) |
14a |
|
| 17 |
33.1CH2 |
1.49 |
m |
13 |
|
| 18 |
|
1.85 |
m |
|
|
| 19 |
143.8C |
|
|
|
|
| 20 |
168.1C |
|
|
|
|
| 21 |
125.5CH2 |
5.51 |
brs |
|
1,15,16 |
| 22 |
|
|
brs |
|
1,15,16 |
| 23 |
29.5CH3 |
1.45s |
|
|
3,4,5 |
| 24 |
17.4CH3 |
1.47s |
|
|
7,8,9 |
| 25 |
15.7 CH3 |
1.30s |
|
|
11,12,13 |
Table 1: Spectra NMR (dC dan dH) of new compoundanticancer from Softcoral Sinulariasp.in CDCl3.
The compound is categorized in membranoid diterpenoid group, and this compound is normally found in soft coral. According to Ching, Ojika et al. and Jian et al. soft coral Sinularia flexibilliis contains secondary metabolites from Membranoid group and has cytotoxic activity toward HeLa cancer cells and MCF-7 [6,14,15]. Most of the soft corals has bioactive compounds from membranoid-diterpenoid groups which are toxic and inflammable [15]. Anticancer activities of the three-new compound of soft coral Sinularia sp can be seen from the high toxicity level of low value of IC50. According to Nursid if the value of IC50 is low, the compound has a potential to be used as anticancer [4]. Soft coral has attracted many researchers because of its diversity in unique chemical structure which is related to its pharmacological activities.
The compound is categorized in membranoid diterpenoid group, and this compound is normally found in soft coral. According to Ching, Ojika et al. and Jian et al. soft coral Sinularia flexibilliis contains secondary metabolites from Membranoid group and has cytotoxic activity toward HeLa cancer cells and MDF-7 [6,14,15]. Most of the soft corals has bioactive compounds from membranoid-diterpenoid groups which are toxic and inflammable [16]. Anticancer activities of the three-new compound of soft coral Sinularia sp can be seen from the high toxicity level of low value of IC50. According to Nursid if the value of IC50 is low, the compound has a potential to be used as anticancer [4]. Soft coral has attracted many researchers because of its diversity in unique chemical structure which is related to its pharmacological activities. Three new compounds are name manadosinularine because Manado is the place of where the coral live and sinularine relates to the soft coral Sinularia sp.
Conclusion
Toxicity activities of isolated compound of soft coral sinularia sp from Bunakan Island Indonesia, is specific toward three tested cancer cells seen from the value of IC50. Three tested compounds are very active on HeLa cancer cell where the IC50 is 0.64 mg/l the molecule formula of is C22H28O5, molecule weight is 348.19. Analysis with LC-MC and NMR showed that is new compound and is name Unsrat-sinularine. The structure of that compound belongs to membranoid diterpenoid group.
Acknowledgements
This research was funded by Research Center of Marine and Fisheries Product Processing and Biotechnology, Marine and Fisheries Research and Development Board, Ministry of Marine Affairs and Fisheries. The authors would like to thank:
1. Dr. Agus Heri (The Head of Marine and Fisheries Research and Development Board, Ministry of Marine Affairs and Fisheries in Jakarta).
2. The Head of Research Center of Marine and Fisheries Product Processing and Biotechnology.
3. Prof. Dr. Ellen J, Kumaat, MSc, Rector Sam Ratulangi University.
4. Prof. Dr. Grevo Gerung, MSc Dean Faculty of Fisheries and Marine Science Sam Ratulangi University.
5. Sri Iswani, Spi, for the help during research, Dewi Fajarningsih, MSc, for the help during bioactive test of the sample to cancer cells.
6. Dr. H. Nursid, MSi, for the help in analysis of tested cells.
7. Dr. Dewi, MSc, for the motivation during the research and Mr Tamrin for the support.
8. Dr. Jeane Mamuaya, MSc and Dr. Rose Mantiri, MSc. to the editor my paper.
20400
References
- Rompas RM (2011) Farmakognosi Laut (Sumber Baru Ekonomi Kelautan).Indonesia.
- Januar HI, Motti CH, Tapiolas D, Wright AD (2009) Analisis Dereplikasi untuk Identifikasi Senyawa Antibakteri Sponge Axinella sp. Dari Perairan Kepulauan Karimunjawa. Jurnal Pescapanen dan Bioteknologi Kelautan dan Perikanan 4: 1-6.
- Pawlik JR (1993) Marine Invertebrate Chemical Defenses.Chemical Reviews93: 1911-1922.
- Nursid M, Dewi Fajarningsih N, Wikanta TH (2009) Isolation of Cytotoxic Compound from Nephtheasp. Soft Coral. Journal of Biotechnology Research in Tropical Region 2: 1.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nicholas DE, et al. (1982) Brine shrimp: a convenient general bioassay for active plantconstituents. Planta Medica45: 31-34.
- Ching Ch, Su W, Chin CH, Wu YJ, Jui HS (2013) Oxygenated Cembranoids from the Soft Coral Sinularia flexibilis. Int J Mol Sci14: 4317-4325.
- Menchetner E, Kyshtoobayeva A, Zonis S, Kim H, Stroup R, et al. (1998) Levels of Multidrug Resistance (MDR1) P-Glycoprotein Expression by Human Breast Cancer Correlate with in Vitro Resistance to Taxol and Doxorubicin. Clinical Cancer Research 4: 389-398.
- Aouali N, Morjani H, Trussardi A, Soma E, Giroux B, et al. (2003) Enhanced Cytotoxicity and Nuclear Accumulation of Doxorubicin-loaded Nanospheres in Human Breast Cancer MCF-7 Cells Expressing MRP1. International Journal of Oncology 23: 1195-1201.
- Zampieri L, Bianchi P, Ruff P, dan Arbuthnot P (2002) Differential Modulation by Estradiol of P-glycoprotein Drug Resistance Protein Expression in Cultured MCF7 and T47D Breast Cancer Cells. Anticancer Res 22: 2253-2259.
- Onuki R, Kawasaki H, Baba T, Taira K (2003) Analysis of a Mitochondrial Apoptotic Pathway Using Bid-Targeted Ribozymes in Human MCF7 Cells in the Absence of a Caspase-3-Dependent Pathway. Antisense and Nucleic Acid Drug Development 13: 75-82.
- Prunet C, Ewing SL, Ménétrier F, Néel D, Lizard G (2005) Activation of Caspase-3-Dependent and -Independent Pathways During 7-Ketocholesterol- and 7ß-Hydroxycholesterol-Induced Cell Death: A Morphological and Biochemical Study. Journal of Biochemical and Molecular Toxicology 19: 311-326.
- Schafer JM, Lee ES, O’Regan RM, Yao K, Jorand VC (2000) Rapid development of tamoxifen-stimulated mutant p53 breast tumors (T47D) in athymic mice. Clin Cancer Res 6: 4373-4380.
- Ojika M, Islam MK, Shintani T, Zhang Y, Okamotoand T (2003) Three new cytotoxic acylspermidines from the soft coral, Sinularia sp. Biosci. Biotechnol. Biochem 67: 1410.
- Jian Y, Min Z, Minshan M, Yuping X, Zheng X, et al. (2013) New Casbane Diterpenoids from a South China Sea Soft Coral, Sinularia sp.Jur Marine Drugs 11: 455-465.
- Huei-Jyun S, Yen-Ju T, Chiung-Yao H, Zhi-Hong W, Chang-Feng D, et al. (2012) Cytotoxic and anti-inflammatory diterpenoids from the Dongsha Atoll soft coral Sinularia flexibilis.Elsevier. Tetrahedron.
- Choi YH, FJ(2012) Cytotoxic acylated Spermidine from a soft coral, Sinularia sp. Journal of Natural Products 60:495-496.
- ZacharyI(2003) Determination of cell number. In: Cell proliferation and apoptosis. Bios Scientific Publishers,pp: 13-35.







