Laura K Markham1* and Milo A Hollingworth2
1University of Bristol Medical School, UK
2North Bristol Trust, UK
*Corresponding Author:
Laura K Markham
64 Princess Victoria Street
Clifton, Bristol, BS8 4DD, UK
Tel: 07701061923
E-mail: lm1132@my.bristol.ac.uk
Citation: Markham LK, Hollingworth MA. De Novo Arteriovenous Malformation in a 4 Year-Old Boy with Headache, with No Previous Cerebrovascular Disease. J Neurol Neurosci. 2016, 6:3. doi: 10.21767/2171-6625.100036
Received Date: October 05, 2015; Accepted Date: October 16, 2015; Published Date: October 19, 2015
Keywords
Cerebral arteriovenous; Malformation
The Case
A 4 year-old boy presented to clinic with a 6-month history of headache. The headaches were severe and localised over the right temporal cranium. The headaches would last 10-15 minutes and triggered crying, causing the boy to lie down until his symptoms resolved. At onset, the headaches were limited to two episodes daily, however their frequency and duration have increased; 3 months prior to his first meeting with the neurosurgical service, headache frequency had increased to 5 times per day before becoming constant in nature. Importantly, the headaches were not associated with photophobia, nausea or vomiting nor had his parents noticed any specific triggers. His mother had treated his symptoms with daily ibuprofen.
The 4 year old was born via normal vaginal delivery after an uncomplicated pregnancy, however during the neonatal period he was reported to have suffered seizures. Head magnetic resonance imaging (MRI) performed at the time revealed no underlying abnormality (Figure 1). The patient was commenced on phenobarbital, which was discontinued at 2 years following complete seizure cessation and he continues to be seizurefree. The boy’s medical and family history were otherwise insignificant, particularly regarding migraines, and there were no developmental concerns.
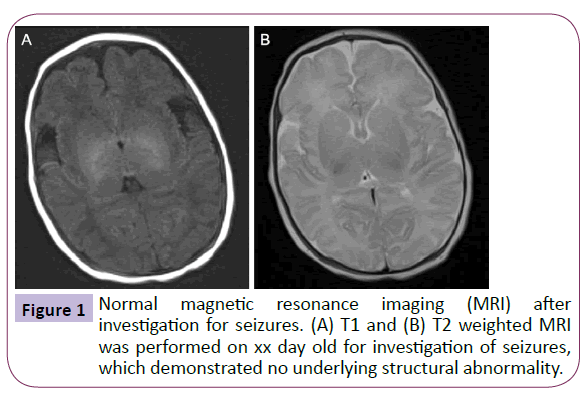
Figure 1: Normal magnetic resonance imaging (MRI) after investigation for seizures. (A) T1 and (B) T2 weighted MRI was performed on xx day old for investigation of seizures, which demonstrated no underlying structural abnormality.
On examination, the patient was neurologically intact with a height, weight and head circumference within the normal range for his age. The patient underwent further head imaging to identify the origin of his new onset headaches. MRI demonstrated a diffuse arteriovenous malformation (AVM) in the posterior portion of the left superior temporal gyrus (Figure 2), which was confirmed by angiography (Figure 3). On later questioning, there was no family history of AVM and after comparison with the MRI performed during infancy, it was confirmed that this patient had acquired an AVM de novo. The patient now awaits surgical management and his care is overseen by neurology services for the management of his headaches.
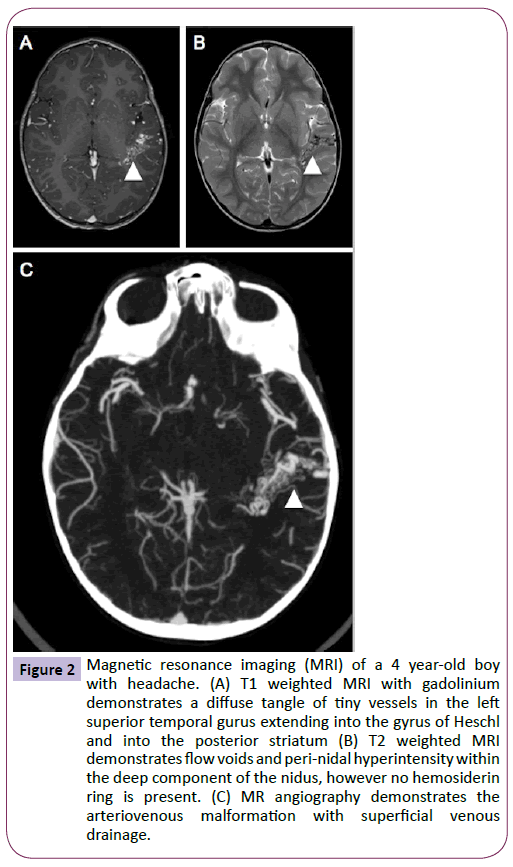
Figure 2: Magnetic resonance imaging (MRI) of a 4 year-old boy with headache. (A) T1 weighted MRI with gadolinium demonstrates a diffuse tangle of tiny vessels in the left superior temporal gurus extending into the gyrus of Heschl and into the posterior striatum (B) T2 weighted MRI demonstrates flow voids and peri-nidal hyperintensity within the deep component of the nidus, however no hemosiderin ring is present. (C) MR angiography demonstrates the arteriovenous malformation with superficial venous drainage.
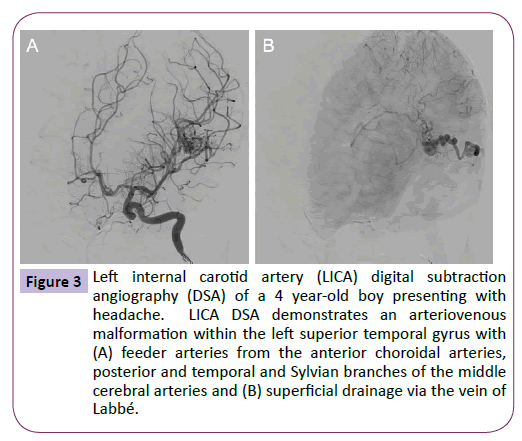
Figure 3: Left internal carotid artery (LICA) digital subtraction angiography (DSA) of a 4 year-old boy presenting with headache. LICA DSA demonstrates an arteriovenous malformation within the left superior temporal gyrus with (A) feeder arteries from the anterior choroidal arteries, posterior and temporal and Sylvian branches of the middle cerebral arteries and (B) superficial drainage via the vein of Labbé.
Discussion
In 1928, Walter Dandy suggested AVMs were derived from enduring embryonic arteriovenous connections that failed to regress after foetal development [1]. Since then, there has been a prevailing view in neurosurgical texts describing AVM formation as a congenital phenomenon [2]. AVMs are thought to originate when the embryo is between 40 and 80 mm in length, during which time processes such as vasculogenesis, angiogenesis, vascular remodelling and differentiation take place [3,4]. It has therefore been widely accepted that cerebral AVMs are present at birth and follow a silent course before becoming clinically evident [5,6].
The case presented herein adds to only 15 reported cases of de novo AVM [7-21] that challenge the concept of congenital aetiology. Furthermore, this case is only the eighth case described in a child without underlying cerebrovascular disease such as moya-moya disease, or a history of previous vascular malformation (Table 1). It has been argued that the appearance of de novo AVM is due to limitations in imaging sensitivity; thrombosis, haemorrhage and oedema can obscure arteriovenous flow visualised by angiography [22]. However, this case and the majority of the previous reported cases demonstrate de novo AVM using sensitive high-resolution cross-sectional imaging such as MRI (Table 1). Importantly, with the increasing availability of MRI, it is likely that we will recognise more cases of de novo AVM in the future.
| Case |
Details of initial presentation and imaging |
Details of presentation with de novo AVM |
| Presentation |
Imaging |
Diagnosis |
Presentation |
Imaging |
Diagnosis |
Management |
| [7] |
16y female
Right facial palsy |
MRI |
Bell’s Palsy |
30y
Chronic migraine |
MRI |
Left frontoparietal AVM |
Unspecified |
| [8] |
15y male
New onset seizures |
MRI |
None |
24y
Seizure |
MRI and DSA |
Left parietal AVM |
Operative resection |
| [9] |
26y female
Multifocal neurological deficit |
MRI |
Unspecified midbrain hyperintensity |
32y
Intracerebral haemorrhage |
CT and MRI |
Right temporal AVM |
Operative resection |
| [10] |
3y female
Trauma |
MRI |
Left frontal intracerebral haemorrhage |
6y
Intractable epilepsy since trauma |
MRI and DSA |
Right temporal lobe AVM |
Operative resection |
| [11] |
6y female Developmental delay and seizure |
MRI |
None |
9y
Increased seizure frequency post-trauma |
MRI and angiogram |
Left temporal-occipital lobe AVM |
Radiation therapy |
| [12] |
25y male
Seizures |
Angiogram |
None |
50y
Seizure and aura of vivid auditory hallucination |
CT |
Large left temporal lobe AVM |
Conservative |
| [13] |
4y male
Seizures |
MRI |
None |
7y
Seizure |
MRI and angiography |
Right occipital lobe AVM |
Operative resection |
| [14] |
10y female
Transient ischaemic attack |
MRI |
Moya-moya disease |
14y
Follow-up |
MRI |
Right occipital lobe AVM |
Conservative |
| [15] |
6y male
Seizures |
MRI |
Cavernous malformation, developmental venous anomaly |
9y
Follow-up |
MRI |
Choroidal AVM |
Unspecified |
| [16] |
3y male
Left hemispheric infarct |
CT and angiogram |
Moya-moya disease |
11y
Follow-up |
MRI |
Left posterior parietal AVM |
Unspecified |
| [17] |
9y female
Seizures |
CT and MRI |
Right parietal AVM treated with Gamma knife type B |
11y
Follow-up |
MRI and angiogram |
Deeper, more medial right parietal AVM |
Radiosurgery |
| [18] |
10y female
Intraventricular haemorrhage |
Angiogram |
Splenium and left occipital lobe AVM managed by gross total resection |
27y
Intracerebral haemorrhage |
MRI |
Midline cingulated gyrus, corpus callosum AVM |
Operative resection |
| [19] |
3y female
Left hemispheric stroke |
MRI |
Sickle cell disease and moya-moya disease |
6y
Follow-up |
MRI |
Right Sylvian region AVM |
Operative resection |
| [20] |
3y male
Seizure |
MRI and DSA |
Right parietal AVM treated with embolisation with four coils and Onyx |
7y
Seizure |
MRI and angiogram |
Left medial occipital AVM |
Embolisation and Onyx |
| [21] |
3 weeks female |
MRI |
Two enhancing extracranial masses |
2.5y
Follow-up |
MRI |
Cerebellar AVM |
Unspecified |
*case with de novo AVM without pre-existing cerebrovascular disease
MRI: Magnetic Resonance Imaging
CT: Computer Tomography
Table 1: Summary of documented cases of de novo arteriovenous malformation (AVM) in patients with and without pre-existing cerebrovascular disease.
In addition to the reports of de novo AVM development, the exceedingly rare diagnosis of cerebral AVMs in utero further questions the assumption that AVMs originate during embryonic development [23-25]. The current standard of prenatal imaging techniques is such that abnormalities such as vein of Galen aneurysmal malformations are frequently detected in utero or in the early post-natal period [26]. This indicates the lack of peri-natal cerebral AVM diagnosis should not be blamed on technological inadequacy, but instead point towards the need for a new understanding of cerebral AVM pathogenesis.
The exact mechanism of cerebral AVM formation remains unclear, though is thought to involve a combination of genetic and environmental factors. The most common genetic abnormality appears to derive from mutations in the gene coding for activin-like kinase 1 (Alk1) [26]. Several studies have found loss-of-function mutations in Alk1 in hereditary haemorrhagic telangiectasia (HHT) type 2 [27]. HHT is an autosomal dominant condition that can present with cerebral AVMs [26]. Mutations in Alk1 affect transforming growth factor-β (TGF-β), which is critical for angiogenesis and inflammation. Alk1 deletion in mouse models is associated with cerebrovascular de novo AVM [28]. Importantly, these AVMs only occurred after stimulation with vascular endothelial growth factor (VEGF) [28]. Indeed, VEGF promotes vascular proliferation and is markedly increased in patients with cerebral AVM [29]. However, VEGF stimulation alone has never been shown to induce de novo AVM formation in animal models.
Similarly to the HHT studies, the genetic predisposition for AVM formation has been studied in familial clustering of sporadic cerebral AVMs. A recent systematic review included all studies reporting single-nucleotide polymorphisms (SNPs) associated with sporadic cerebral AVMs and describes a statistically significant association between an SNP in the ALK1 gene and a susceptibility to developing cerebral AVMs (OR 2.19, 95% CI 1.25- 3.83) [26,30].
One proposed mechanism for AVM formation extrapolates the genetic predispositions demonstrated by animal models and illustrates an important potential interaction between primary genetic mutations and the cerebrovascular microenvironment in the formation of AVM. It is thought asymptomatic parenchymal venous thrombosis triggers local hypertension and ischaemia [26]. This in turn triggers uncontrolled vascular proliferation and ultimately arteriovenous shunt formation. The presence of the aforementioned genetic susceptibility, as well as abnormalities of the angiogenesis and inflammatory cascades prevents these processes from being self-limited [26]. Once the vascular lesion is subjected to high flow, endothelial shear stress contributes to a continued angiogenic stimulus and a hyperangiogenic environment. These phenomena can also explain the documented propensity of AVMs to increase and decrease in size spontaneously [31,32] and in some cases, dramatically recur years after AVM surgery, gamma knife and embolisation [33,34].
Conclusions
We describe a case of de novo AVM in a patient without previous cerebrovascular disease. The dynamic nature of cerebral AVM has been previously described, however, considering this further case of reported de novo AVM and recent animal models, we emphasize the need to re-examine the traditional ideas regarding AVM pathogenesis.
Conflict of Interest
The authors declare no conflict of interest
7361
References
- Dandy WE (1928) Venous abnormalities and angiomas of the brain. Arch Surg 17: 715-793.
- Yasargil MG (1987) Pathological considerations, in: Microneurosurgery: AVM of the Brain, Embryology, Pathologic Considerations, Hemodynamics, Diagnostic Studies, Micro-surgical Anatomy. New York: Thieme Verlag 3A: 49-211.
- Davidson AS, Morgan MK (2011) The embryologic basis for the anatomy of the cerebral vasculature related to arteriovenous malformations. J Clin Neurosci 18: 464-469.
- Mullan S, Mojtahedi S, Johnson DL, Macdonald RL (1996) Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg 85: 1-8.
- Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, et al. (2003) Prospective, population-based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke 34: 1163-1169.
- Stapf C, Mast H, Sciacca RR, Berenstein A, Nelson PK, et al. (2003) The New York Islands AVM Study: design, study progress, and initial results. Stroke 34: e29-e33.
- Mahajan A, Manchandia TC, Gould G, Bulsara KR (2010) De novo arteriovenous malformations: case report and review of the literature. Neurosurg Rev 33.
- Neil JA, Li D, Stiefel MF, Hu YC (2014) Symptomatic de novo arteriovenous malformation in an adult: Case report and review of the literature. Surg Neurol Int 5: 148.
- Bulsara KR, Alexander MJ, Villavicencio AT, Graffagnino C (2002) De novo cerebral arteriovenous malformation: case report. Neurosurgery 50: 1137-1141.
- Gonzalez LF, Bristol RE, Porter RW, Spetzler RF (2005) De novo presentation of an arteriovenous malformation. Case report and review of the literature. J Neurosurg 102: 726-729.
- Stevens J, Leach JL, Abruzzo T, Jones BV (2009) De novo cerebral arteriovenous malformation: case report and literature review. American Journal of Neuroradiology 30: 111-112.
- Ozsarac M, Aksay E, Kiyan S, Unek O, Gulec FF (2012) De novo cerebral arteriovenous malformation: Pink Floyd's song "Brick in the Wall" as a warning sign. Journal of Emergency Medicine 43: 17-20.
- Wu J, Li Y, Cao Y, Wang S (2014) De novo cerebral arteriovenous malformations: is epileptic seizure a potential trigger? Childs Nerv Syst 30: 1277-1281.
- Fujimura M, Kimura N, Ezura M, Niizuma K, Uenohara H, et al. (2014) Development of a de novo arteriovenous malformation after bilateral revascularization surgery in a child with moyamoya disease. J Neurosurg Pediatr 13: 647-649.
- Alvarez H, Perry V, Solle M, Castillo M (2012) De novo cerebral arteriovenous malformation in a child with previous cavernous malformation and developmental venous anomaly. J Neurosurg Pediatr 9: 327-330.
- Schmit BP, Burrows PE, Kuban K, Goumnerova L, Scottt RM (1996) Acquired cerebral arteriovenous malformation in a child with moyamoya disease. Case report. J Neurosurg 84: 677-680.
- Rodriguez AC, Martinez R, Rey G, Bravo G (2000) Recurrence in a different location of a cerebral arteriovenous malformationin a child after radiosurgery. Childs Nerv System 16: 363-365.
- Akimoto H, Komatsu K, Kubota Y (2003) Symptomatic de novo arteriovenous malformation appearing 17 years after the resection of two other arteriovenous malformations in childhood: case report. Neurosurgery 52: 228-232.
- O’Shaughnessy BA, DiPatri AJ Jr, Parkinson RJ, Batjer HH (2005) Development of a de novo cerebral arteriovenous malformation in a child with sickle cell disease and moyamoya arteriopathy. Case report. J Neurosurg 102: 238-243.
- Bai Y, He C, Zhang H, Ling F (2012) De novo multiple dural arteriovenous fistulas and arteriovenous malformation after embolization of cerebral arteriovenous fistula: case report. Childs Nerv Syst. 28: 1981-1983.
- Song JK, Niimi Y, Kupersmith MJ, Berenstein A (2007) Postnatal growth and development of a cerebral arteriovenous malformation on serial magnetic resonance imaging in a child with hemangiomatosis. Case report. Journal of Neurosurgery 106: 384-387.
- Gonzalez LF, Bristol RE, Porter RW, Spetzler RF (2005) De novo presentation of an arteriovenous malformation. Case report and review of the literature. J Neurosurg 102: 726-729.
- Auyeung KM, Laughlin S, Terbrugge KG (2003) Prenatal diagnosis of unusual fetal pial arteriovenous malformation. A case report. Interv Neuroradiol 9: 163-168.
- DeCesare B, Omojola MF, Fogarty EF, Brown JC, Taylon C (2006) Spontaneous thrombosis of congenital cerebral arteriovenous malformation complicated by subdural collection: in utero detection with disappearance in infancy. Br J Radiol 79: e140-e144.
- Eguchi S, Aihara Y, Yamaguchi K, Okada Y (2012) Limitations of fetal ultrasonography and magnetic resonance imaging in prenatal diagnosis of congenital cerebral arteriovenous malformations with hemorrhagic onset. Case report. J Neurosurg Pediatr 10: 154-158.
- Morales VSF, Bortholottie C, Sturiale C, Lanzino G (2014) Are parenchymal AVMs congenital lesions? Neurosurgical Focus 37.
- Johnson DW, Berg JN, Baldwin MA, Gallione CJ, Marondel I, et al. (1996) Mutations in the activin receptor-like kinase 1 gene in hereditary haemorrhagic telangiectasia type 2. Nat Genet 13: 189-195.
- Chen W, Sun Z, Han Z, Jun K, Camus M, et al. (2014) De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 45: 900-902.
- De novo cerebrovascular malformation in the adult mouse after endothelial Alk1 deletion and angiogenic stimulation. Stroke 45: 900-902.
- Sturiale CL, Puca A, Sebastiani P, Gatto I, Albanese A, et al. (2013) Single nucleotide polymorphisms associated with sporadic brain arteriovenous malformations: where do we stand? Brain 136: 665-681.
- Ezura M, Kagawa S (1992) Spontaneous disappearance of a huge cerebral arterio-venous malformation: case report. Neurosurgery 30: 595-599.
- Minakawa T, Tanaka R, Koike T (1989) Angiographic follow-up study of cere-bral arteriovenous malformations with reference to their enlargement and regression. Neurosurgery 24: 68-74.
- Gabriel EM, Sampson JH, Wilkins RH (1996) Recurrence of a cerebral arteriovenous malformation after surgical excision: case report. J Neurosurg 84: 879-882.
- Kader A, Goodrich JT, Sonstein WJ (1996) Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg 85: 14-18.








