Keywords
Colon cancer; Endoscopy; Fecal occult blood test; Decision analysis; Stool DNA test; Genetic testing
Clinical Practice Points
What is already known about this subject?
Colonoscopy has the highest sensitivity and specificity to detect cancer, precancerous lesions, and non-advanced adenoma in colon cancer screening as a gold standard. Stool DNA test provides higher sensitivity and specificity than FIT. We also know the probability of adverse events for colonoscopy and FSIG.
What are the new findings?
The sDNA has the highest integrated utility for patients, based on sensitivity, specificity, patients’ utility, probability of adverse events, and mortality. If we modify the integrated utility with participation rate and the participation rate of sDNA is higher than 60.3%, sDNA will still have the highest integrated utility than other approaches.
How might it impact on clinical practice in the foreseeable future?
Based on the evidence from this study, the sDNA should be recommended to average-risk population as a regular approach for colon cancer screening, in order to derive higher integrated utility and thus replace previous invasive approaches.
Background
Colorectal cancer, also known as colon cancer, rectal cancer, bowel cancer or colorectal adenocarcinoma, is a neoplastic problem in colon or rectum, or in the appendix. Typical symptoms of colorectal cancer include rectal bleeding, anemia, weight loss and changes in bowel habits. Colorectal cancer is the third most common cancer in the world; approximately 60% cases were diagnosed in developed countries. It is estimated that worldwide 1.23 million new cases of colorectal cancer were clinically diagnosed, and 608,000 people died of this type of cancer in 2008 [1].
Common risk factors of colorectal cancer are increasing age, male gender [2], high intake of fat, alcohol or red meat, obesity, smoking and a lack of physical exercise [3]. Only a small proportion of colon cancer cases, approximately 5%-25%, are due to genetic risk [3].
The risk of colon cancer increases sharply since age of 50 [4]. Regular colon cancer screening, starting from 50 until 75, is an effective way decreasing the chance of dying from this neoplastic disease, and thus highly recommended by health care provider in developed countries [5]. Currently regular screening approaches are colonoscopy and sigmoidoscopy.
According to national comprehensive cancer network (NCCN) colon cancer guideline, “average risk” group for colon cancer should take regular colon cancer screening program every 5 years or 10 years, with accordance with detailed requirements in different countries. We could summarize the decision options as follows.
Decision options
The NCCN "Guidelines for Colon Cancer Screening" recommends, beginning at age 50, both men and women should follow one of these testing schedules for screening to find colon polyps and cancer [6]:
• Colonoscopy (COL)
• Flexible sigmoidoscopy (FSIG)
• Guaiac fecal occult blood test (gFOBT), and
• Fecal immunochemical test (FIT), also called immunochemical fecal occult blood test (iFOBT)
According to recommendations made by American College of Gastroenterology Guidelines, people have two more traditional options for entire colon examination.
• Air contrast barium enema (ACBE), and
• Computed tomographic colonography (CTC)
However, these two approaches are dominated by COL [7]. Thus, we only take COL into consideration among three entirecolon- examination methods (COL, ACBE and CTC), as an option for decision analysis on screening approaches.
Besides, with the development of health testing technology, patients now have one more choice, a brand-new testing for colon cancer [8]:
• Stool DNA testing for colon cancer (sDNA)
Patients may know little about these available options on their efficacy and burden. Thus all the approaches in consideration will be introduced in the following, which serve as background information for decision analysis for colon cancer screening.
The most common approach is colonoscopy. It is the endoscopic examination of the large bowel and the distal part of the small bowel with a CCD camera or a fibre optic camera on a flexible tube passed through the anus [9]. A colonoscopy allows an examination of the entire colon (1200–1500 mm in length). It can provide a visual diagnosis for ulceration and polyps, and opportunities to remove polyps and suspected colorectal cancer lesions. Once polyps are removed, they will be examined whether they are precancerous or not, in laboratory with the aid of microscope and electronic equipment. Basically colonoscopy provides the most precise approach for screening participants as the sensitivity and specificity for adenoma and colon cancer are highest among all available approaches [10].
The second testing is sigmoidoscopy, which is also an endoscopic examination method. There are two types of sigmoidoscopy, one with flexible tube and one with rigid tube [11]. The recommended method in NCCN is flexible sigmoidoscopy, usually abbreviated as FSIG. FSIG enables physician to observe the inside of the large intestine from the rectum through the last part of the colon, called the sigmoid, and any abnormal phenomena in sigmoid, including intestinal bleeding, inflammation, abnormal growths, and ulcers. Physicians could use the procedure to find the cause of diarrhea, abdominal pain, or constipation. They also use it to look for benign and malignant polyps in sigmoid, as well as early signs of cancer. However, FSIG is not enough to detect polyps or cancer in the ascending or transverse colon (twothirds of the colon), though it is useful in descending colon where colon disease happens most frequently [12]. For anything unusual observed in sigmoid, like a polyp or inflamed tissue, the physician can remove a piece of the tissue and send it to lab for further testing.
The third method is guaiac fecal occult blood test (gFOBT), detecting the presence of occult blood in stool. The term “guaiac” denotes the paper surface used in the test which has alpha-guaiaconic acid, extracted from a special kind of trees [13]. In the test feces are applied to the guaiac paper and smeared to be fecal sample film. After that, one or two drops of chemical liquid (hydrogen peroxide) are dripped on the film, to observe the speed of color change. If there is no fecal occult blood, the color changes slowly; otherwise it changes fast, indicating there is probability of colorectal problem. Therefore, a positive test result is one where there is a quick and intense blue color change of the film. However, this method is considered complicated, as it requires a strict fasting from iron supplements, red meat, certain vegetables, Vitamin C, and citrus fruits for a period of time before the test [14]. The restriction actually limits the application of the technique, but it is still considered sufficient for average risk people, to effectively reduce the mortality associated with colon cancer [15].
The fourth method is fecal immunochemical test (FIT), also called immunochemical fecal occult blood test (iFOBT), which also detects hidden occult blood within the stool as gFOBT. This detection is important because it can be a sign of precancerous polyps or colon cancer. FIT is done essentially the same way as gFOBT, though it may be found easier than gFOBT since there is no drug or dietary restrictions. FIT also requires as few as only one stool sample, rather than three in gFOBT. FIT is more effective in terms of health outcomes and cost compared with gFOBT [16]. However, both FIT and gFOBT are not enjoyable experiences, as either the patients or screening professionals take complicated steps to collect stool samples, which causes discomfort and embarrassment [17].
The latest approach for colon cancer screening is stool DNA testing (sDNA), designed to identify recognizable DNA changes (DNA markers) in cells that are continually shed from the lining of the colon through stool. Stool samples and those cells affiliated in stool are collected, and thus changes in DNA markers in cells could be identified, if there is presence of precancerous polyps or colon cancer. Because DNA changes may differ between colon cancers, stool DNA tests typically target multiple markers to achieve high detection rates [18]. Stool DNA testing shows to be more effective than fecal occult blood tests (gFOBT and FIT) at detecting colon cancer and precancerous polyps [8].
Many previous studies have elaborated the pros and cons of different colon cancer screening methods, and compared them clinically. A randomized controlled trial showed that subjects in the FIT group were more likely to participate in screening than were those in the colonoscopy group, while more adenomas were identified in the colonoscopy group than were in the FIT group [19]. Another RCT also stated that the attendance rate of colonoscopy group was significantly lower compared with FSIG and FIT groups [20]. A population-based randomized trial compared FIT and gFOBT, and found participation and detection rates for advanced adenomas and cancer were significantly higher for FIT group than gFOBT group; gFOBT significantly underestimated the prevalence of advanced adenomas and cancer in the screening population [21]. It has been concluded that different screening methods have different utilities, techniques, and clinical performances, thus leads to various attendance rates and detection rates. Hence, we need to consider all these factors and possible results as well, in order to compare different colon cancer screening methods.
Whatever option patient may choose, they might have four possible results generally: colon cancer, precancerous lesions, non-advanced adenoma, and completely negative results.
Colon cancer has already been introduced above. Once a participant was diagnosed as colon cancer, he or she should immediately receive a colectomy or chemotherapy, or both. Colectomy is a surgical procedure to remove all or part of the patient’s colon [22]. Chemotherapy for colon cancer may be used at different times during the entire treatment procedure. Chemotherapy may be used after surgery to remove the cancer, which is known as adjuvant chemotherapy. For some cancers, chemotherapy is given (sometimes with radiation) before surgery to shrink the cancer and make surgery easier. For advanced colon cancers, chemotherapy can also be used to help shrink tumors and relieve symptoms for cancers that have spread to other organs [23].
Common drug combinations used for chemotherapy include [24,25]:
• FOLFOX: 5-FU, leucovorin, and oxaliplatin,
• CapeOx: Capecitabine and oxaliplatin,
• 5-FU and leucovorin,
• Capecitabine
However, chemotherapy has side effects for patients, including hair loss, mouth sores, loss of appetite, nausea and vomiting, or low blood counts. Types of side effects depend on the type and dosage of chemotherapy drugs given to patients and the length of time they are taken.
Precancerous lesions refer to adenomatous polyps (adenomas) that have the potential to become cancer. Patients are usually recommended to take screening to find the precancerous lesions and make lesions in the colon removed through polypectomy or colectomy [26,27].
Non-advanced adenoma refers to benign clumps of cells (polyps) in colon, which is less likely to become cancer. Physicians usually recommend observing for a period of time, or removing the adenoma by polypectomy [28-30].
Methods
Data extraction
All variables applied in this decision analysis were listed in Table 1 (Appendix 1). Data were extracted from related peerreviewed articles, and carefully examined. The efficacy of colonoscopy is very high. The sensitivities are 77%, 98%, and 98% respectively for detecting low-risk adenoma, intermediate /high-risk adenoma, and colon cancer; the specificity of colonoscopy for adenoma and colon cancer is 97%. Colonoscopy provides a golden standard for colon cancer screening based on its high efficacy, though it causes pains, discomfort, and embarrassment for patients.
| Variable |
Value |
Range for SA |
Distribution |
Source |
| Low |
High |
Type |
Alpha |
Beta |
| Screening Test Characteristics? |
| COL Sensitivity |
[31] |
| for low-risk adenomas |
77.000% |
73.000% |
80.000% |
Beta |
350 |
104.55 |
[32] |
| for intermediate/high-risk distal adenomas |
98.000% |
93.000% |
99.000% |
Uniform |
93.0% |
99.0% |
| for CRC |
98.000% |
95.000% |
99.000% |
Uniform |
95.0% |
99.0% |
| COL Specificity |
|
[31] |
| for adenomas and CRC |
97.000% |
96.000% |
98.000% |
Beta |
970 |
30 |
| FSIG Sensitivity |
|
|
|
|
|
|
[33] |
| for low-risk distal adenomas |
65.000% |
60.000% |
70.000% |
Beta |
235 |
126.54 |
|
| for intermediate/high-risk distal adenomas |
74.000% |
68.000% |
78.000% |
Beta |
180 |
63.24 |
|
| for distal CRC |
90.000% |
85.000% |
95.000% |
Beta |
90 |
100 |
|
| FSIG Specificity |
|
|
|
|
|
|
[33] |
| for distal adenomas and CRC |
92.000% |
90.000% |
95.000% |
Beta |
250 |
21.74 |
|
| gFOBT Sensitivity |
|
[34] |
| for adenomas |
10.300% |
10.000% |
12.000% |
Beta |
11.4 |
92.1 |
[35] |
| for cancer |
36.000% |
31.000% |
42.000% |
Beta |
105 |
186.6 |
[36] |
| gFOBT Specificity |
|
|
|
|
|
|
[37] |
| for adenomas and cancer |
97.000% |
96.000% |
98.000% |
Beta |
1083.4 |
33.5 |
[38] |
| FIT Sensitivity |
|
[36] |
| for adenomas |
21.000% |
19.000% |
22.000% |
Beta |
594.62 |
2236.92 |
[39] |
| for cancer |
71.000% |
67.000% |
75.000% |
Beta |
35.29 |
143.08 |
[40] |
| FIT Specificity |
|
[39] |
| for adenomas and cancer |
95.000% |
94.000% |
96.000% |
Beta |
1732.57 |
91.19 |
|
| sDNA Sensitivity |
|
[8] |
| for adenomas |
17.200% |
15.900% |
18.600% |
Gamma |
60 |
0.0103 |
Calculation* |
| for cancer |
92.300% |
83.000% |
97.500% |
Gamma |
498 |
0.00023 |
Calculation* |
| sDNA Specificity |
|
|
|
|
|
|
[8] |
| for adenomas and cancer |
89.800% |
88.900% |
90.700% |
Gamma |
4002 |
0.00015 |
Calculation* |
| Adverse Events |
| COL Probability |
? |
[41] |
| Perforation with polypectomy |
0.216% |
0.168% |
0.298% |
Uniform |
0.168% |
0.298% |
[42] |
| Perforation without polypectomy |
0.107% |
0.010% |
0.249% |
Uniform |
0.010% |
0.249% |
|
| Death following Perforation |
5.195% |
0.000% |
0.907% |
Uniform |
0.000% |
0.907% |
|
| Bleeding |
0.379% |
0.065% |
0.412% |
Uniform |
0.065% |
0.412% |
|
| FSIG Probability |
|
[43] |
| Perforation |
0.002% |
0.000% |
0.051% |
Uniform |
0% |
0.05% |
[42] |
| Death following Perforation |
6.452% |
0.000% |
9.070% |
Uniform |
0.000% |
9.070% |
| Bleeding |
0.029% |
0.002% |
0.054% |
Uniform |
0.002% |
0.054% |
| Utility |
| Cancer free |
0.94 |
|
[44] |
| CRC |
0.8 |
0.43 |
0.94 |
0.94*Beta |
3.92 |
0.69 |
[45] |
| Stage-specific utility |
|
[46] |
| for stage I |
0.74 |
- |
- |
- |
- |
- |
- |
| for stage II |
0.67 |
- |
- |
- |
- |
- |
- |
| for stage III |
0.5 |
- |
- |
- |
- |
|
- |
| for stage IV |
0.25 |
- |
- |
- |
- |
- |
- |
| Mortality |
| Annual CRC-specific mortality |
? |
[47] |
| for stage I |
0.014 |
- |
- |
- |
- |
- |
- |
| for stage II |
0.0377 |
- |
- |
- |
- |
- |
- |
| for stage III |
0.0986 |
- |
- |
- |
- |
- |
- |
| for stage IV |
0.3951 |
- |
- |
- |
- |
- |
|
| Natural mortality for any case (age 65) |
0.0126 |
- |
- |
- |
- |
- |
[48] |
| Participation |
| COL |
0.86 |
0.81 |
0.9 |
Uniform |
0.81 |
0.9 |
[49] |
| FSIG |
0.39 |
0.24 |
0.67 |
Uniform |
0.24 |
0.67 |
[50] |
| gFOBT |
0.53 |
0.32 |
0.7 |
Uniform |
0.32 |
0.7 |
| FIT |
0.53 |
0.32 |
0.7 |
Uniform |
0.32 |
0.7 |
| % never participate |
0.13 |
0 |
0.41 |
|
?- |
?- |
Abbreviations: COL=colonoscopy, FSIG= Flexible sigmoidoscopy, gFOBT=Guaiac fecal occult blood test, FIT=Fecal Immunochemical Test, sDNA=stool DNA testing, CRC=colorectal cancer.*Distribution and related parameters are decided and calculated by author of this study. The method is introduced in interpretation part.
Table 1: Parameters for decision analysis.
Flexible Sigmoidoscopy provides second best efficacy for colon cancer screening. The sensitivities are 65%, 74%, and 90% respectively for detecting low-risk adenoma, intermediate /high-risk adenoma, and colon cancer; the specificity of FSIG for adenoma and colon cancer is 92%. The sensitivity and specificity for colon cancer is pretty high, though this approach might be more possible to falsely suggest nonexistence of low/intermediate/high risk adenoma than colonoscopy.
The gFOBT has a really high specificity, 97%, for adenoma and colon cancer. However, it performs poorly in sensitivity for adenoma and colon cancer, which are 10.3% and 36% respectively, indicating that this approach probably is lacking the ability in detecting adenoma and colon cancer.
The FIT provides a high specificity, 95%, for adenoma and colon cancer, which is approximately the same as gFOBT. The sensitivity of FIT for adenoma and colon cancer is higher than those of gFOBT, which are 21% and 71%, respectively. Both FIT and gFOBT causes no pains for patients, but their performance in detecting adenoma and colon cancer is not ideal, compared with invasive approaches. The advantage of these two noninvasive methods is that patient could take screening annually to make up the inefficiency, though it might be costly.
The Stool DNA testing performs much better in detecting colon cancer, as the sensitivity is 92.3%. The sensitivity for adenoma is slightly lower than that of FIT, which is 17.2%. The specificity for adenoma and colon cancer is as high as 89.8%, though slightly lower than FIT. The brand-new approach could also be applied annually, and provides much higher utility than invasive screening methods, as there is no pains and discomfort.
Invasive screening methods might cause adverse events, including bleeding, perforation, or even death. Though the probabilities of these adverse events of colonoscopy and FSIG are low, which are all below 7%, they indeed influence patients’ utility largely.
The utility of cancer free status is 0.94, and utility of colon cancer is 0.8, which are derived from two highly cited studies. The utility of colon cancer is a comprehensive utility, and synthesized many cases, including different stages and different treatments, recovering from colon cancer, and recurrence. The stage-specific utility of colon cancer is lower than the comprehensive utility, because it didn’t integrate cases comprehensively, and targeted at a different population group. Stage-specific utilities are still kept in Table 1 for further consecutive study.
Mortalities of stages of colon cancer come from a well-cited study. The definition of stages of colon cancer comes from National Cancer Institute [28]. Annual mortality is derived from 5-year survival rate of each stage. Mortalities are 0.014, 0.0377, 0.0986, and 0.3951 respectively for stage I, II, III and IV. The natural mortality for any case is collected from Canada Life Table. As DNA clinical trial recruited participants between 50 and 84, with weighted mean age of 65, we use Canadian natural mortality at age of 65 for analysis, which is 0.0126.
Finally we collected participation rate of different screening approached to modify the efficacy for average risk population screening, as only the participants could have benefits brought by screening approaches. The participation rate of colonoscopy is as high as 86%; 53% of people participated in FIT or gFOBT; only 39% of people participated in FSIG; and there is 13% of people who had never attended any screening program.
This study adopts a perspective of a third payer. The confidence interval and distribution information for each variable was reserved in order to perform probabilistic sensitivity analysis (PSA) in further study, though PSA was not performed in this study.
Decision tree
We integrated both colon cancer screening and treatment into our decision analysis to build a comprehensive decision tree and the structure of the decision tree is shown in the Figure 1.
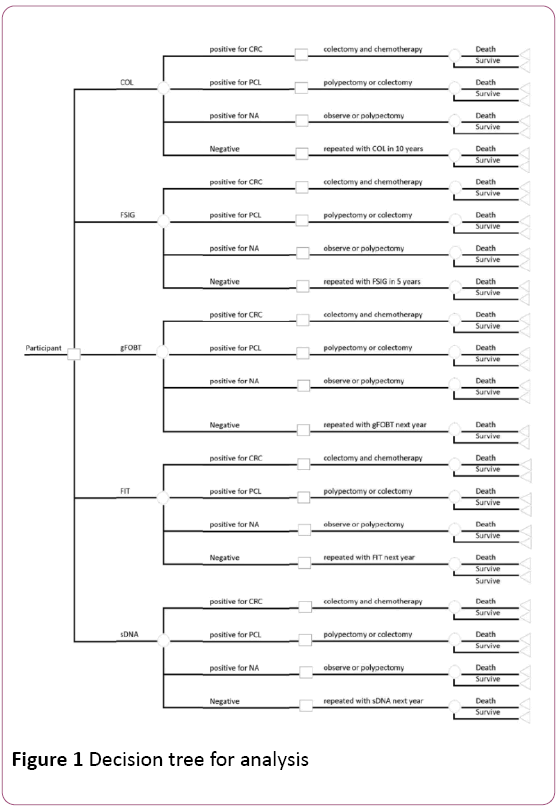
Figure 1: Decision tree for analysis
When a participant plans to attend colon cancer screening, he might have five options, which are COL, FSIG, gFOBT, FIT, and sDNA. Different screening methods have different utility and efficacy. For each type of examination, there are four possible results, which are colon cancer, precancerous lesions, non-advanced adenoma, and negative results. For different results, different treatments are assigned. After treatment, patient has a probability to survive or die, with two different utilities assigned to two statuses respectively.
Assumptions
Before this decision tree was applied to analyze the decision question faced by hundreds of thousands of people in the average-risk group for colon cancer, there are several important assumptions that have been made explicitly.
Firstly we assumed the mortality of each status after screening, including colon cancer, precancerous lesions, nonadvanced adenoma, and negative results. Since there is no recent study on colorectal precancerous lesions mortality, we assigned mortality of stage I colon cancer to the status of precancerous lesions. This assumption is made based on expert opinion. As non-advanced adenoma could be easily removed by polypectomy, or even left for further observation, we assume there is no significant difference between mortality of nan-advanced adenoma and natural mortality. Besides, we also assumed these mortalities are conditional probabilities given patients have received proper treatment.
The utilities of screening approaches are assumed based on existing studies on embarrassment and discomfort survey of COL, FSIG, gFOBT, and FIT. As for sDNA, we assumed it provides perfect utility for patients, as it is non-invasive and very easy to collect stool samples. Compared with sDNA, the utilities of gFOBT and FIT are both assumed at 0.8, as the sample collection of FIT/gFOBT is more complicated than sDNA method, and caused embarrassment and discomfort. The utility of FSIG is assumed at 0.25, as much more subjects in a survey reported FSIG caused embarrassment and discomfort than FIT and gFOBT [17]. Colonoscopy provides average utility of 0.5 to patients, as in another survey the number of complaints on discomfort caused by FSIG was approximately twice as that number caused by colonoscopy [29].
The prevalence of colorectal cancer, precancerous lesions, non-advanced adenoma, and negative results, are derived from the NEJM study, in which 9989 average-risk participants were screened, with 65 diagnosed as colon cancer, 757 as precancerous lesions, 2893 as non-advanced adenoma, and the rest as negative results and non-neoplastic findings [8]. The prevalence of each status in general population is not appropriate to be applied in this study, as people below 50 rarely take colon cancer screening, and thus they are not subjects of interest (Figure 2).
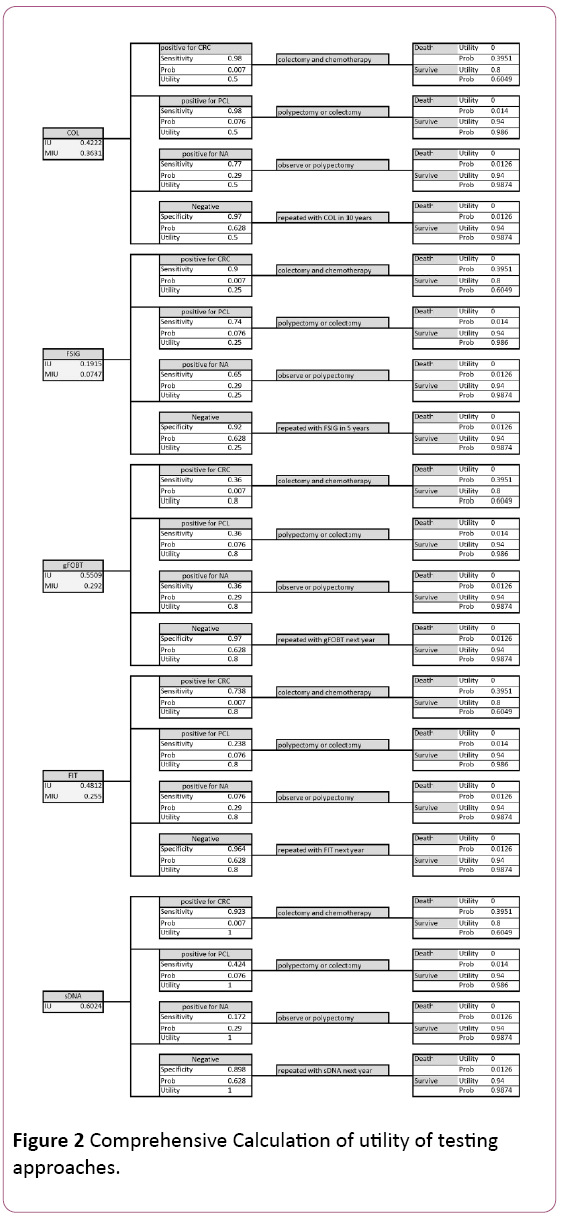
Figure 2: Comprehensive Calculation of utility of testing approaches.
We also reasonably assume FSIG sensitivity for precancerous lesions as 0.36, because the sensitivities for colorectal cancer and non-advanced adenoma are both 0.36.
Results
The aim of this study is to compare utilities of different screening approaches. The utility should be considered and calculated from two parts: the burden of the test itself, and the results of treatment. The reason why we should consider treatment is that treatment utility is influenced by test efficacy. Thus we calculated the integrated utility for each approach.
The decision tree integrated with screening and treatment shows sDNA has the highest integrated utility, 0.6024, given the probability and utility of each disease status and related treatment, and the ability to detect the four statuses. The gFOBT provides second best integrated utility of 0.5509; the utility of FIT is 0.4812, followed by 0.4222 for colonoscopy and 0.1915 for FSIG.
The integrated utilities above should be modified by participation rate of each approach. After modification, colonoscopy performs better than gFOBT and FIT, as the modified integrated utility is 0.3631, higher than 0.292 for gFOBT and 0.255 for FIT. The FSIG still performs worst, with a modified integrated utility at 0.0747. As a brand-new screening method, sDNA has no participation rate record. However, from results above we could predict that sDNA will have a better modified integrated utility if the hypothetical participation rate is higher than 60.3%.
Sensitivity Analysis
In this study we performed one-way sensitivity analysis. From Table 2 we could find the integrated utility of sDNA is sensitive to variations of specificity of negative results, test utility, and participation rate.
| Input parameter |
Range |
Influence on sDNA integrated utility rank |
Threshold Value |
| Low |
High |
| Test sensitivity for CRC |
0.36 |
1 |
No change |
- |
| Test sensitivity for PCL |
0.36 |
1 |
No change |
- |
| Test sensitivity for NA |
0 |
1 |
No change |
- |
| Test specificity for Negative |
0.8 |
1 |
Change the rank within the range |
>0.81* |
| Test utility |
0.8 |
1 |
Change the rank within the range |
>0.915* |
| Participation rate |
0.53 |
1 |
Change the rank within the range |
>0.603* |
*Values greater than threshold ensure sDNA has the highest integrated utility.Abbreviation: CRC=colorectal cancer, PCL=precancerous lesions, NA=non-advanced adenoma, chemotherapy=capecitabine or 5FU/leucovorin, IU=Integrated utility, MIU=Modified integrated utility.
Table 2: One-way sensitivity analysis.
We set a variation range from 0.8 to 1 for test specificity of negative results. As all the other approaches perform well in specificity, it is reasonable to set this range. If the specificity were less than 0.81, sDNA would perform worse than gFOBT on integrated utility, given other parameter remained.
For test utility of sDNA, a variation range from 0.8 to 1 was built up. As it is a non-invasive method with simple stool sample collecting procedure, the utility of sDNA could not be less than FIT and gFOBT. Thus it is reasonable to set this range for test utility. This approach will perform worse than gFOBT, the current second best, if the test utility is less than 0.915.
Integrated utility is most sensible to participation rate. We assume the lower bar for participation rate variation is 0.53, which is the participation rate for gFOBT and FIT, and upper bar is 1. If the rate remains larger than 0.603, sDNA will prove to be best test in term of integrated utility.
Variation on sensitivity for colon cancer, precancerous lesions, and non-advanced adenoma will not affect the fact that sDNA performs best. Variation on mortality and utility of disease status was not considered, as it is not directly related with test approach. Besides, prevalence of disease statuses was assumed to be stable.
Conclusion
The presented clinical evidence shows that sDNA provides highest integrated utility for people in screening program. It should be recommended for average-risk population to take this brand-new approach annually. The integrated utility is sensitive to specificity for negative results, test utility, and test participation rate.
Discussion
This study integrated both screening stage and treatment stage together to explore the evidence for colon cancer screening decision analysis. However, it currently considered only efficacy and utility of test and treatment, without integration of cost data. Hence, cost should be considered and incorporated to explore the cost-effectiveness of sDNA as a brand-new population-based screening approach.
This study was subject to some important limitations. Firstly, the sensitivity and specificity of sDNA comes from a single source, in which the study was conducted in North America. If the context changes, it might be possible that both the sensitivity and specificity will change. Secondly, there was no probabilistic sensitivity analysis, which is more informative than one-way sensitivity analysis. Thirdly, the model was developed from the perspective of a third-party payer, such as a provincial ministry of health, the organization that decides on funding for a governmental screening program for colorectal cancer. For this reason, lost productivity costs, which are necessary to determine the societal perspective, were not incorporated. Finally, this study only considers people with average risk, thus it is less likely to generalize the conclusion to high-risk population.
10866
References
- Ferlay SH, Bray F, Forman D, Mathers C, Parkin DM (2010) GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer 2010.
- Cunningham AW, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, et al. (2010). Colorectal cancer. Lancet 375: 1030–1047.
- Watson AJ, (2011) Colon cancer: a civilization disorder. Digestive diseases (Basel, Switzerland) 29: 222-228.
- Howlader NA, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al. (2012) SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations)". National Cancer Institute, Bethesda, MD, USA.
- Society CC (2014) Colon cancer screening. https://www.cancer.ca/en/prevention-and-screening/early-detection-and-screening/screening/screening-for-colorectal-cancer/?region=pe 2014.
- Rockey D, Paulson E, Niedzwiecki DE (2005) Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. The Lancet 365: 305-311.
- Imperiale TF, Ransohoff DF, Itzkowitz SH (2014) Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med 370: 1287-1297.
- Wikipedia. Colonoscopy. https://en.wikipedia.org/wiki/Colonoscopy 2014.
- Rex DK, Rahmani EY, Haseman JH (1997) Relative sensitivity of colonoscopy and barium enema for detection of colorectal cancer in clinical practice. Gastroenterology 112: 17-23.
- Beg M (2002) Occult Gastrointestinal Bleeding: Detection, Interpretation, and Evaluation. JIACM 3: 153-158.
- Bretthauer M (2010) Evidence for colorectal cancer screening. Best Pract Res Clin Anaesthesiol 24: 417-425.
- Rex DK, Johnson DA, Anderson JC (2009) American College of Gastroenterology guidelines for colorectal cancer screening 2008. The Am J Gastroenterol 104: 739-750.
- Hol L, de Jonge V, van Leerdam ME (2010) Screening for colorectal cancer: comparison of perceived test burden of guaiac-based faecal occult blood test, faecal immunochemical test and flexible sigmoidoscopy. Eur J Cancer 46: 2059-2066.
- Quintero E, Castells A, Bujanda L (2012) Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 366: 697-706.
- Segnan N, Senore C, Andreoni B (2007) Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 132: 2304-2312.
- van Rossum LG, Van Rijn AF, Laheij RJ (2008) Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 135: 82-90.
- Gill S, Thomas R, Goldberg R (2003) Colorectal cancer chemotherapy. Alimentary pharmacology & therapeutics 18: 683-692.
- Kuebler JP, Wieand HS, O'Connell MJ (2007) Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol25: 2198-2204.
- Conteduca V, Sansonno D, Russi S (2013) Precancerous colorectal lesions (Review). Int J Oncol43: 973-984.
- Canadian Cancer Society. Precancerous conditions of the colon and rectum. 2014.
- Zubarik R, Ganguly E, Benway D (2002) Procedure-related abdominal discomfort in patients undergoing colorectal cancer screening: a comparison of colonoscopy and flexible sigmoidoscopy. Am J Gastroenterol 97: 3056-3061.
- Gillespie T (2006) 52 year old woman with colon cancer case study. John Hopkins Advanced Studies in Nursing 4: 118-119.
- van Rijn JC, Reitsma JB, Stoker J (2006) Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol 101:343-350.
- Bressler B, Paszat LF, Chen Z (2007) Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology 132:96-102.
- Lieberman DA, Harford WV, Ahnen DJ (2001) One-time screening for colorectal cancer with combined fecal occult-blood testing and examination of the distal colon. N Engl J Med 345:555-560.
- Allison JE, Feldman R, Tekawa IS (1990) Hemoccult Screening in Detecting Colorectal Neoplasm: Sensitivity, Specificity, and Predictive ValueLong-Term Follow-up in a Large Group Practice Setting. Annals of internal medicine 112:328-333.
- Castiglione G, Grazzini G, Poli A (1991) Hemoccult sensitivity estimate in a screening program for colorectal cancer in the Province of Florence. Tumori 77:243.
- Allison JE, Tekawa IS, Ransom LJ (1996) A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 334:155-160.
- Brevinge H, Lindholm E, Buntzen S (1997) Screening for colorectal neoplasia with faecal occult blood testing compared with flexible sigmoidoscopy directly in a 55–56 years' old population. Int J Colorectal Dis 12:291-295.
- Collins JF, Lieberman DA, Durbin TE (2005) Accuracy of screening for fecal occult blood on a single stool sample obtained by digital rectal examination: a comparison with recommended sampling practice. Annals of internal medicine 142:81-85.
- Allison JE, Sakoda LC, Levin TR (2007) Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst99:1462-1470.
- Cheng TI, Wong JM, Hong CF (2002) Colorectal cancer screening in asymptomatic adults: comparison of colonoscopy, sigmoidoscopy and fecal occult blood tests. J Formos Med Assoc101:685-690.
- Dafnis G, Ekbom A, Pahlman L (2001) Complications of diagnostic and therapeutic colonoscopy within a defined population in Sweden. Gastrointestinal endoscopy 54:302-309.
- Gatto NM, Frucht H, Sundararajan V (2003) Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst95:230-236.
- Atkin WS, Cook C, Cuzick J (2002) Single flexible sigmoidoscopy screening to prevent colorectal cancer: baseline findings of a UK multicentrerandomised trial. Lancet 359:1291-1300.
- Fryback DG, Lawrence WF (1997) Dollars may not buy as many QALYs as we think: a problem with defining quality-of-life adjustments. Medical Decision Making 17:276-284.
- Ramsey SD, Andersen MR, Etzioni R (2000) Quality of life in survivors of colorectal carcinoma. Cancer 88:1294-1303.
- Ness RM, Holmes AM, Klein R (1999) Utility valuations for outcome states of colorectal cancer. Amjgastroenterol 94:1650-1657.
- O’Connell JB, Maggard MA, Ko CY(2004) Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst96:1420-1425.
- Vernon SW (1997) Participation in colorectal cancer screening: a reviewJ Natl Cancer Inst89:1406-1422.
- Mandelson MT, Curry SJ, Anderson LA (2000) Colorectal cancer screening participation by older women. Am J Prev Med 19:149-154.








