Key words
|
| |
| Simvastatin, PLGA, Pleuronic F-68, 3² factorial design, sustained release |
| |
INTRODUCTION
|
| |
| Drug low solubility and stability in physiological environment constitutes a main hurdle in attaining the appropriate bioavailability. Several polymerbased nanotechnologies are being intended in order to optimize the technological (e.g., solubility, stability, bioavailability, etc.) aspects of drugs. Among them, polymeric nanoparticles, dendrimers, polymeric micelles and polymersomes appear as the most attractive and promising.[1,2] Simvastatin have been described as the principal and most effective class of drugs to reduce serum cholesterol levels, and shown to significantly reduce cardiovascular events and mortality in patients with or without coronary artery disease. The different types of statins are available, Atorvastatin, Cerivastatin, Lovastatin, Pravastatin and Simvastatin. Its molecular weight is 418.57 and the empirical formula is C25H38O5. Simvastatin, a crystalline compound, is practically insoluble in water and hence poorly absorbed from the GI tract. It is a potent and specific inhibitor of 3- hydroxy-3-methyl-glutaryl coenzyme A (HMG CoA) reductase, which catalyzes the reduction of HMG CoA to mevalonate. Thus, simvastatin arrests a key step for cholesterol biosynthesis in liver, and is hence widely used in the treatment of hypercholesterolemia and dyslipidemia, as an adjunct to diet. After oral administration, simvastatin is metabolized to its β- dihydroxy acid form (simvastatin acid) by the cytochrome-3A system in liver, where it inhibits the rate-limiting step in cholesterol biosynthesis.[3] |
| |
| Being a Class II drug, it often shows dissolution rate limited oral absorption and high variability in pharmacological effects. Therefore, improvements in its solubility and/or dissolution rate may lead to enhancement in bioavailability. Various attempts to enhance the dissolution rate and bioavailability of simvastatin have been reported. Like that preparation of nanoparticles, various methods, currently used are precipitation, high pressure homogenization and pearl milling, either in water or in mixtures of water and water-miscible liquids or nonaqueous media.[4,5] Nanoprecipitation is a technique, where a drug solution in a water miscible organic solvent is mixed with an aqueous solution containing a surfactant. Upon mixing, the supersaturated solution leads to nucleation and growth of drug particles, which may be stabilized by surfactants.[6] |
| |
| The aim of present work was formulation of nanoparticles by nanoprecipitation method and find out the effect of stabilizer on the formulation, when all parameters of operation are kept constant. Formulation of nanoparticles using Polylactide coglycolide as lipophilic polymer, simvastatin as a model drug and Pleuronic F- 68 as surfactant stabilizer and evaluated with different parmeters such as particle size, % encapsulation efficiency, in vitro drug release, fourier transform infra red study, scanning electron microscopy and differential scanning colorimetric study.[7,8] |
| |
MATERIALS AND METHODS
|
| |
| Simvastatin was a obtained from gift sample from Aurobindo Pharmaceutical Ltd., Hydrabad; Poly (D, L Lactide-co- Glycolide) (PLGA 50:50) was obtained as gift samples from Purac biochem ltd. Netherland; Pluronic F 68 was purchased from sigma chemicals, Mumbai, dialysis bag (cellophane membrane, molecular weight cut off 10000-12000 Da, purchased from Hi-Media, Mumbai. India. All other reagents and chemicals used in this study were of analytical Grade. |
| |
|
Methods
|
| |
|
Formulation of poly (lactic-co-glycolic acid) (PLGA) Nanoparticles:
|
| |
| PLGA nanoparticles were prepared by the nanoprecipitation - solvent deposition method.[9] Simvastatin was dissolved in acetone. Hydrophilic stabilizer Pleuronic F68 dissolved in 40 ml of water. PLGA was solubilized in acetone 20ml and add simvastatin solution at various concentrations. The organic phase was poured into the aqueous solution drop wise, with syringe positioned with the needle directly into stabilizer containing water under stirring at 3000 RPM for 2 hrs, thus forming a milky colloidal suspension. The organic solvent was then evaporated by using a Rota evaporator. The resultant dispersion was dried using a freeze drying method.[10] |
| |
|
Experimental Design:[11]
|
| |
| The formulations were fabricated according to a 32 full factorial design, allowing the simultaneous evaluation of two formulation variables and their interaction. The experimental designs with corresponding formulations are shown in table no.1.& 2. The dependent variables that were selected for study were particle size (Y1) and % drug entrapment (Y2). |
| |
|
In Vitro Characterization of PLGA Nanoparticles Determination of particle size:
|
| |
| The particle size and size distribution of the simvastatin loaded PLGA (50:50) nanoparticles were characterized by photon correlation spectroscopy (PCS) using a Zetasizer 2000 Malvern Instruments, UK. Nanosuspension was diluted with filtered (0.22μm) ultra pure water and analysed using Zeta sizer. This analysis yields the mean diameter (zaverage, measuring range: 20–1000 nm), which allows sample measurement in the range of 0.020- 2000.00 μm. [12] |
| |
|
Determination of Encapsulation Efficiency: Estimation of Free Drug: [13]
|
| |
| The free drug was estimated by taking 100mg of formulation in dialysis bag, which was tied and placed into 100ml of Phosphate buffer pH 6.8 kept at 37°C on magnetic stirrer. At predetermined time intervals, 5ml of the samples were withdrawn by means of a syringe. The volume withdrawn at each interval was replaced with same quantity of fresh Phosphate buffer pH6.8 maintained at 37ºC. The samples were analyzed for free drug by measuring the absorbance at 238nm using UV-visible spectrophotometer, Shimadzu UV-1700, after suitable dilution. Above described process of withdrawing sample and analysis was continued till a constant absorbance was obtained. |
| |
|
Estimation of encapsulated drug: [14]
|
| |
| Encapsulated drug was estimated by taking residue formulation remained in dialysis membrane after estimation of free drug content, as described above. Quantity left behind in dialysis membrane was added to acetone 10ml to dissolve PLGA and filtered. Residue remaining on filter paper was dissolved in 100ml of phosphate buffer pH 6.8 kept at 37 ºC and after removing supernatant, sample was analyzed for drug content by measuring the absorbance at 238 nm using UV-visible spectrophotometer Shimadzu UV- 1700 after suitable dilution. The percentage of drug entrapped and the percentage of free drug are calculated by following Equations, Formulae to calculate % free drug and % drug entrapment, |
| |
 |
| |
 |
| |
| Polydispersity index: Polydispersity was determined according to the equation, Polydispersity index = D (0.9)-D (0.1)/ D (0.5) Where, D (0.9) corresponds to particle size immediately above 90% of the sample. D (0.5) corresponds to particle size immediately above 50% of the sample. D (0.1) corresponds to particle size immediately above 10% of the sample |
| |
|
Statistical Analysis: [15]
|
| |
| The results from factorial design were evaluated using PCP Disso 2000 V3 software. Stepwise backward linear regression analysis was used to develop polynomial equations for dependent variables particle size (Y1) and % drug entrapment (Y2) which form of equation-1: |
| |
| Y= β0 + β1X1 + β2X2 + β11X12 + β22X22 + β12X1X2 + ε … 1 |
| |
| Where Y is estimate response of dependent variable, β0 arithmetic mean response of nine batches, and β1 estimated coefficient for factor X1. The main effects (X1 and X2) represent average result of changing one factor at a time from its low to high value. The interaction term (X1X2) shows how the response changes, when two factors are simultaneously changed. The polynomial terms (X12 and X22 ) are included to investigate non-linearity. ε is the random error. The simplified models were then utilized to produce three dimensional response surface plots to analyze the influence of independent variables. |
| |
|
In vitro drug Release Study:
|
| |
| A quantity of selected factorial formulations equivalent to 25 mg of the drug, table no.1 & 2 indicates drug content per 100mg of formulation was taken in the dialysis bag. The dialysis bag was then suspended in a flask containing 100 ml of Phosphate buffer pH 6.8 on a magnetic stirrer at 37± 0.5ºC at 100 rpm. Required quantity 5ml of the medium was withdrawn at specific time periods ( 0.5,1, 2, 3 ,4, 6 , 8,10, 12, 24 hours) and same volume of dissolution medium was replaced in the flask to maintain a constant volume. The withdrawn samples were filtered through a filter paper (0.22 μm, Whatman Inc., USA) and 5 ml filtrate was made up to volume with 100 ml of Phosphate buffer pH 6.8. The samples were analyzed for drug release by measuring the absorbance at 238 nm using UV-visible spectrophotometer and calculate percent cumulative release of simvastatin. [16] |
| |
|
Fourier Transform Infrared Spectroscopy (FTIR) study:
|
| |
| Infrared spectrum of simvastatin, nanoparticle formulation was determined by using Fourier Transform Infrared Spectrophotometer (FTIR-4100, Shimadzu) using KBr dispersion method. The base line correction was done using dried potassium bromide. Then the spectrum of dried mixture of drug and potassium bromide was run. [17] |
| |
|
Differential scanning calorimetry (DSC) study:
|
| |
| Differential scanning calorimetry (DSC) is one of the most powerful analytical techniques, which offers the possibility of detecting chemical interaction of Simvastatin, PLGA and simvastatin nanoparticles. DSC measurements were carried out on a modulated DSC Instrument: SDT Q600 V20.9 Build 20 equipped with a thermal analysis data system (TA instrument). Samples of 2–10 mg were placed in aluminium pans and sealed. The probes were heated from 25 to 250ºC at a rate of 10 K/min under nitrogen atmosphere. [18,19] |
| |
|
X-ray Diffraction Study:
|
| |
| X-ray diffraction analysis was employed to detect the crystallinity of the pure drug and the nanoparticle formulation, which was conducted using a Philips PW 3710 x-ray diffractometer (XRD) with a copper target and nickel filter (Philips Electronic Inst, Holland). Powders were mounted on aluminium stages with glass bottoms and smoothed to a level surface. The XRD pattern of each sample was measured from 10 to 50 degrees 2-theta using a step increment of 0.1 2- theta degrees and a dwell time of 1 second at each step. [20] |
| |
|
Scanning electron microscopy (SEM) study:
|
| |
| The morphology of nanoparticles was examined by using scanning electron microscopy (SEM, JSM-6360LV scanning microscope Tokyo, Japan). The nanoparticles were mounted on metal stubs using double-sided tape and coated with a 150 Å layer of gold under vacuum. Stubs were visualized under scanning electron microscope. SEM has been used to determine particle size distribution, surface topography, texture and examine the morphology of fractured or sectioned surface. The same generally used for generating three dimensional surface relief images derived from secondary electrons. The examination of surface of polymeric drug delivery system. [21] |
| |
RESULT AND DISCUSSION
|
| |
| All the factorial formulations developed by the nanoprecipitation Solvent disposition method, formulations, were found to be free flowing and white and powdery in appearance. |
| |
|
Particle Size and Entrapment Efficiency:
|
| |
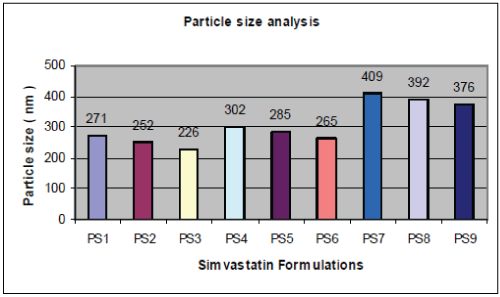
| A graphical representation of the particle size of PLGA nanoparticles obtained is shown in fig. no.1. Particle size is an important parameter because it has a direct relevance to the stability of the formulation. The amount of stabilizer used also has an effect on the properties of nanoparticles. If the concentration of stabilizer is too low, aggregation of the polymer will take place, whereas, if too much stabilizer is used, drug incorporation could be reduced as a result of the interaction between the drug and stabilizer. The effect of the concentration of the polymer, increasing the concentration causes the emulsion to have larger droplets, hence leading to larger particles. From Fig.1 and Fig. 2 and Table-1, it is revealed that as Simvastatin:PLGA ratio increased from 1:1 to 1:2, particle size increased significantly and drug entrapment also increased but there after, further increase in drug : polymer ratio showed, reduced drug entrapment efficiency. |
| |
| This can be explained by observing drug entrapment efficiency of factorial formulations PS1, PS4, PS7, where drug: polymer ratio increased from 1:1, 1:1.5 and 1:2 respectively with constant concentration of Pluronic F68 of 100 mg. Drug entrapment efficiency increased from 78.23%, 81.40% and 97.18%. It is also observed that as percentage of stabilizer increased from 100 mg to 200 mg, entrapment efficiency and particle size decrease significantly. The same can be explained with respective to factorial formulation PS1, PS4, PS7 and PS2,PS3, PS5, PS6, PS8, where it is observed that as drug: polymer ratio increases, entrapment efficiency increased significantly but further increase in drug: polymer ratio has negative or insignificant effect on drug entrapment. For factorial formulation PS7, PS8, PS9, where drug: polymer ratio is constant i.e.1:2 and concentration of stabilizer decreased from 200 mg to 100 mg drug entrapment efficiency increased from 87.03% to 97.18 % and particle size increased from 376nm to 409nm. Thus it can be concluded that the stabilizer had greater influence on both dependent parameters particle size and drug entrapment as compared to drug: polymer ratio. |
| |
|
Polydispersity index:
|
| |
| The Polydispersity index of the data revealed that the particle size distributions of all the formulations are uniform, the results shown in table 1& 3. |
| |
|
In-Vitro Drug release study:
|
| |
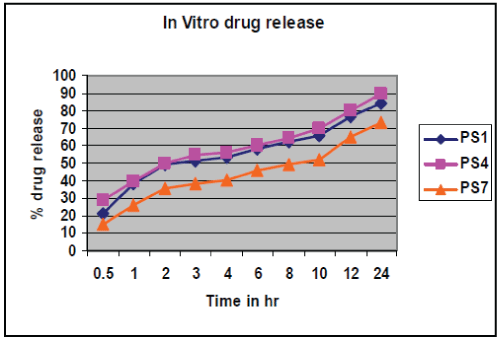
| In vitro drug release study conducted according to highest % drug entrapment and lowest particle size below 271 nm batches PS1, PS4, and PS7 were selected, shown in table no.4.& fig no.3. Drug release from nanoparticles and subsequent biodegradation are important for developing successful formulations. The release rate of nanoparticles by diffusion and biodegradation process. It is generally anticipated from a bulk eroding polymer such as 50:50 PLGA to give an initial burst release followed by a controlled release, in contrast to the release pattern observed in other controlled release systems. |
| |
| The drug release follows first order release kinetics with fickian diffusion mechanism. Drug release for selected factorial formulations PS1, PS4, PS7 are 38.17 %, 39.39 % and 26.19 % respectively, after 1 hr. These formulation show initial burst release followed by a controlled release. Kinetic exponent ‘n’ for these formulations indicate diffusion through the nanoparticle matrix as well matrix erosion. Finally, it can be concluded that the different drug release rates may be attributed to different sizes of the nanoparticles. It is expected as the particle size of PLGA nanoparticle is smaller, their surface area will be more and the drug release is faster. |
| |
|
Development of Polynomial Equations:
|
| |
| The experimental design and Parameters in table no.1 & 2 for factorial formulations PS1 to PS9, polynomial equations for two dependent variables, particle size and % drug entrapment have been derived using PCP Disso 2000V3 software. The equation derived for particle size is: |
| |
| Y1 = 284.46 + 70.93 X1 – 19.166 X2 + 36.600 X12– 0.700X22 + 2400 X1 X2…. 2 |
| |
| The equation derived for % drug entrapment is: |
| |
| Y2 = 75.9087+ 11.4247X1 – 9.128X2 + 4.978 X12–1.9310 X22 + 2.8520 X1X2…. 3 |
| |
| In equations no. 2 negative sign for coefficient of X2 indicates that the particle size of nanoparticles increases, when concentration of stabilizer Pluronic F68 is decreased and positive sign for coefficient of X1 indicate positive effect of concentration PLGA on particle size. In equation no.3, positive sign for coefficient of X1 indicates that the % drug entrapment increases, when concentration of PLGA increases and negative sign for coefficient of X2 indicates that % drug entrapment of nanoparticles increases, when concentration of stabilizer Pluronic F 68 decreases. The closeness of predicted and observed values for particle size and % drug entrapment indicates validity of derived equations for dependent variables. |
| |
|
Response Surface Plots:
|
| |
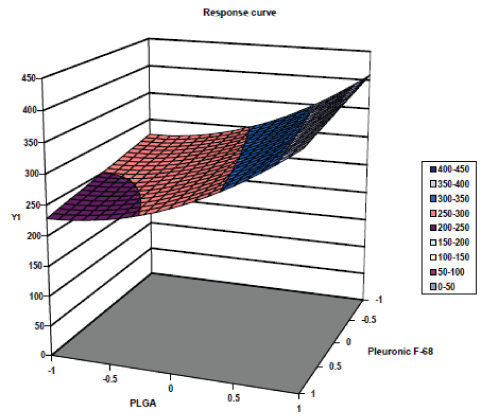
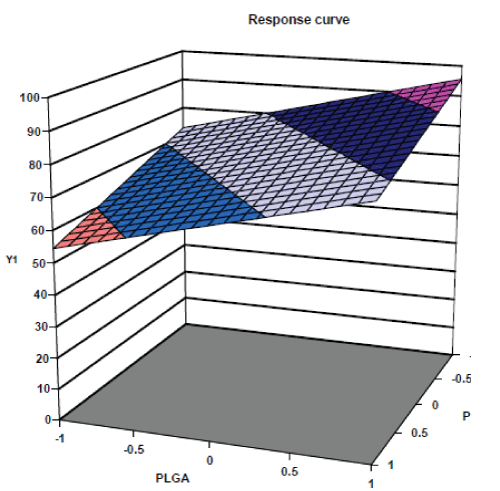
| The response surface plots of particle size and % drug entrapment are shown in fig. no. 4 & 5 respectively. The response surface plots illustrated that as concentration of PLGA increases, the value of dependent variable, particle size increases and as concentration of Pluronic F 68 increases the value of dependent variable, particle size decreases. [22,23] Similarly the response surface plots for % drug entrapment shows positive effects of independent variable, PLGA concentration and negative effect of other independent variable, concentration of Pluronic F 68. |
| |
|
Fourier Transform Infrared Spectroscopy ( FTIR ) study :
|
| |
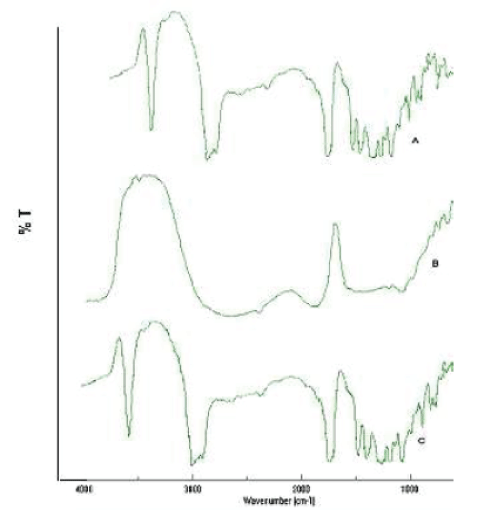
| F.T.I.R. study was carried out to confirm the compatibility between the selected polymer PLGA, drug simvastatin and nanoparticles, are presented in fig. no. 6. The spectra obtained from the I.R. studies are from 3600cm-1 to 400cm-1. It was confirmed that there are no major shifting as well as no loss of functional peaks between the spectra of drug, polymer and drug loaded nanoparticles (1265cm-1, 1380cm-1, 1458cm-1, 1765cm-1, 2968cm-1, 3652cm- 1.) |
| |
|
Differential Scanning Calorimetry (DSC) study:
|
| |
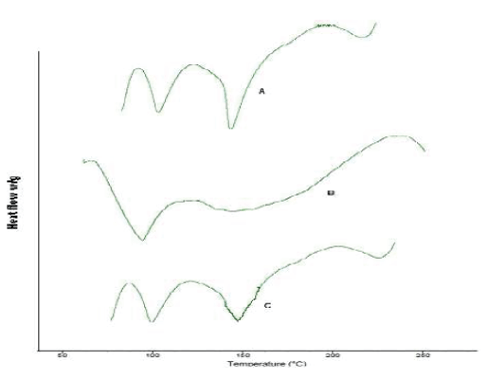
| Differential Scanning Calorimetry study gives information regarding the physical properties like crystalline or amorphous nature of the samples. The DSC thermogram of simvastatin fig. 7-A, shows an exothermic peak at 143.99 ºC corresponding to its melting temperature. However no sharp endotherm was seen at 149.99°C for the DSC curves of the simvastatin nanoparticles in fig 7-C. This shows the crystallinity of the drug has been reduced significantly in the nanoparticles. Hence it could be concluded that in both the prepared PLGA nanoparticles the drug was present in the amorphous phase and may have been homogeneously dispersed in the PLGA matrix. |
| |
|
XRD study:
|
| |
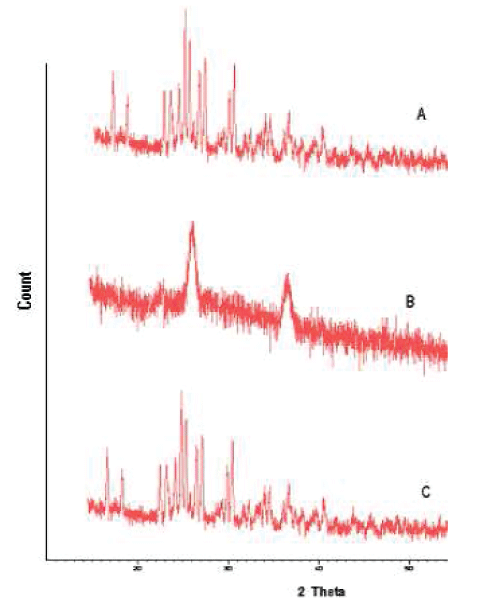
| XRD pattern of the simvastatin, PLGA and selected nanoparticle formulation are shown in fig 8. Characteristic diffraction peaks were observed for commercial simvastatin. On the other hand, the nanoparticles prepared with PLGA was characterized by less intensity of the diffraction peak when compared to that of simvastatin. This clearly indicates the reduction in the crystallanity of the precipitated simvastatin nanoparticles. |
| |
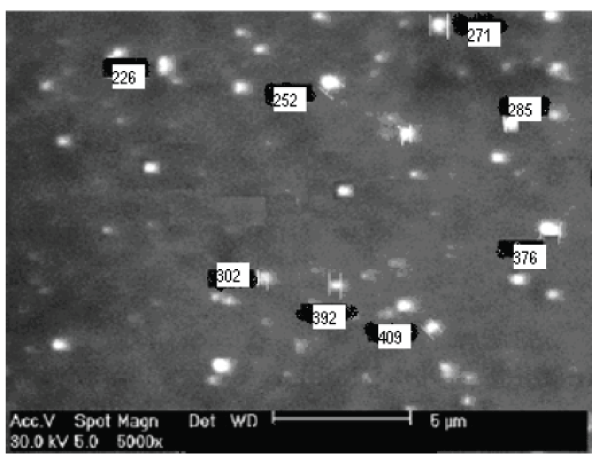
| Scanning electron microscopy (SEM) study: The morphology of nanoparticles was examined by scanning electron microscopy (SEM, JSM-5310LV scanning microscope Tokyo, Japan. The external morphological study using SEM revealed that all nanoparticles were spherical in shape shown in fig. no.9. |
| |
CONCLUSION
|
| |
| Simvastatin loaded nanoparticles were prepared by the nanoprecipitation- solvent disposition method. Drug: polymer ratio and concentration of stabilizer were found to influence the particle size and entrapment efficiency of simvastatin loaded PLGA nanoparticles but the concentration of stabilizer had greater influence on both dependent variables, Particle size and % Drug entrapment as compared to Drug : Polymer ratio. In vitro drug release study of selected factorial formulations PS1, PS4, PS7 showed, 84.56%, 89.65 %, and 73.46 % release respectively in 24 hrs. The release was found to follow first order release kinetics with fickian diffusion mechanism for all batches. So, it conclude that simvastatin loaded PLGA nanoparticles could be effective in sustaining drug release. |
| |
ACKNOWLEDGEMENTS
|
| |
| Authors are wish to acknowledge Aurobindo Pharmaceutical Ltd. (Hydrabad, India), for providing simvastatin as gift sample. We also grateful to Bharati vidyapeeth, Poona college of pharmacy, Pune for providing Malvern Mastersizer facility, Shivaji university Kolhapur for SEM, DSC & XRD facilities and Purac biochem, Netherland for providing, Polylactic co- glycolic acid as gift samples. Author also thank to principal Dr. H. N. More Bharati Vidyapeeth, College of Pharmacy, Kolhapur for providing excellent facility to carry out this work. |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
 |
 |
 |
 |
| Figure 6 |
Figure 7 |
Figure 8 |
Figure 9 |
|
| |

















