Keywords
Repaglinide, HPMC, Sodiumalginate, Carbopol 934P, Buoyancy, Swelling index, In-vitro release.
Introduction
Diabetes Mellitus (DM) is a group of syndromes and chronic metabolic disorder characterized by hyperglycemia, altered metabolism of lipids, carbohydrates and proteins because of a lack of, or ineffective use of the hormone insulin and associated with reduced life expectancy, significant morbidity due to specific diabetes related microvascular complications and diminished quality of life. A fasting blood glucose level of 126 mg /dl and 200mg/dl post prandial (oral Glucose load) is considered as indication of DM [1].
Gastroretentive drug delivery is an approach to prolong gastric residence time, thereby targeting site-specific drug release in the upper gastrointestinal tract (GIT) for local or systemic effects. Gastroretentive dosage forms can remain in the gastric region for long periods and hence significantly prolong the gastric retention time (GRT) of drugs. The use of HPMC, sodium alginate, Carbopol polymer matrices has become extremely popular in controlling the release rate of drugs from solid dosage forms. These systems are attractive from economic as well as process development view point. Floating tablet generally contains mixture of polymer and sodium bicarbonate. Sodium bicarbonate particles provide buoyancy by carbon dioxide bubble formation whereas gel matrix of polymer tries to entrap carbon dioxide bubbles.
Repaglinide lowers blood glucose by stimulating the release of insulin from the pancreas. It achieves this by closing ATP-dependent potassium channels in the membrane of the beta cells. This depolarizes the beta cells, opening the cells calcium channels, and the resulting calcium influx induces insulin secretion. Its short half-life (1hr), dosing frequency (two to four times a day) and local action in the stomach make repaglinide an ideal candidate for floating drug delivery system [2-4].
Materials and Methods
Repaglinide was gift sample from Sun pharma.Pvt. Ltd., Mumbai. Hydroxypropylmethylcellulose K100M was gifted from Colorcon Ltd., Goa. Sodiumalginate, Carbopol 934P and Sodium bicarbonate was purchased from S.D Fine Chemicals, Mumbai. All other ingredients used were of analytical grades.
Preparation of repaglinide floating tablets:
The floating tablets of repaglinide were prepared by direct compression method using 12 mm flatfaced punch of 10 station Rimek compression machine employing different grades of HPMC polymers like K4M, K15 and K100M, Sodium alginate and Carbopol 934P. For the preparation of floating tablets, the active ingredient was thoroughly mixed with polymer(s), diluents and gas forming agent (sodium bicarbonate) using a mortar and pestle for 10 min, magnesium stearate and talc were added to the above blend as flow promoters. In all the formulations, the amount of repaglinide was kept constant at 9.0 mg. The percentage of polymer used was 40% of total tablet weight irrespective of the type of polymer used. The formulae of different matrix tablets of repaglinide are given in the Table 1.
| Sl. No |
Ingredients (mg) |
F1 |
F2 |
F3 |
F4 |
F5 |
| 1 |
Repaglinide |
9 |
9 |
9 |
9 |
9 |
| 2 |
HPMC K4M |
80 |
-- |
-- |
--- |
--- |
| 3 |
HPMC K15M |
--- |
80 |
--- |
---- |
---- |
| 4 |
HPMC K100M |
--- |
--- |
80 |
--- |
--- |
| 5 |
Sodium alginate |
--- |
--- |
--- |
80 |
--- |
| 6 |
Cabopol 934P |
--- |
--- |
---- |
---- |
80 |
| 7 |
Citric acid |
10 |
10 |
10 |
10 |
10 |
| 8 |
Sodium bicarbonate |
28 |
28 |
28 |
28 |
28 |
| 9 |
PVP K30 |
8 |
8 |
8 |
8 |
8 |
| 10 |
MCC |
59 |
59 |
59 |
59 |
59 |
| 11 |
Mg. Stearate |
4 |
4 |
4 |
4 |
4 |
| 12 |
Talc |
2 |
2 |
2 |
2 |
2 |
| 13 |
Total |
200 |
200 |
200 |
200 |
200 |
Table 1: Formulae of repaglinide floating tablets
Dose calculation:
For sustained drug release up to 24 hrs, the total dose of drug required was calculated based on the fact that the conventional dose was 0.5 mg. The total dose was calculated using the following equation,
Dt = Dose (1 + 0.693 × t/t1/2)
Where, Dt = total dose, Dose = Immediate release dose,
t = total time period for which sustained release is required,
t1/2 = half-life of drug [5].
For repaglinide floating tablet:
Dt = 0.5 [1+ (0.693 × 24)/1)]
Dt = 9.0 mg.
Evaluation of Repaglinide Floating Tablets
General appearance
Morphological characters like shape, colour and texture were determined visually.
Thickness
Thicknes of prepared tablets were tested using verniercalipers. The test was done in triplicates and average was determined.
Hardness
The hardness of prepared tablets was determined by using Monsanto hardness tester and measured in terms of kg/cm2. Test was done in triplicate [6].
Weight variation
Twenty tablets were selected randomly from the lot and weighed individually to check for weight variation. IP limit for weight variation in case of tablets weighing 130-324 mg is ± 7.5% and more than 324 mg is ± 5% [7].

Friability
Friability of the tablets was determined using Roche friabilator. This device subjects 10 tablets to the combined effect of abrasions and shock in a plastic chamber revolving at 25 rpm and dropping the tablets at a height of 6 inches in each revolution. Preweighed sample of tablets was placed in the friabilator and were subjected to 100 revolutions. Tablets were dedusted using a soft muslin cloth and reweighed. The friability (F) was calculated by the following formula.
F = (1- W0 / W) × 100
Where, W0 is the weight of the tablets before the test and W is the weight of the tablet after the test [8].
Drug Content
The drug content was carried out by weighing 10 tablets from each batch and calculated the average weight. Then the tablets were triturated to get a fine powder. From the resulting triturate, powder was weighed accurately which is equivalent to 100 mg of repaglinide and dissolved in a 100 ml volumetric flask containing 50 ml of 0.1N hydrochloric acid (HCl) and volume was made up to 100 ml with same solvent. The volumetric flask was shaken for 15 mins and after suitable dilution with 0.1N hydrochloric acid, the drug content was determined using UV-Visible Spectrophotometer at 243 nm [7].
Swelling studies
The extent of swelling was measured in terms of % of weight gained by the tablet. One tablet from each formulation was weighed and kept in Petri dish containing 50 ml of 0.1N Hydrochloric acid solution. At the end of specified time intervals tablets were withdrawn from Petri dish and excess buffer blotted with tissue paper and weighed. The % of weight gained by the tablet was calculated by using following formula:

Where, Mt – weight of tablets at time‘t’; M0 – weight of tablets at time ‘0’[9].
Buoyancy lag time determination and Total floating time
The in vitro buoyancy was determined by the Buoyancy lag time. The tablets were placed in a 250 ml beaker containing 0.1N hydrochloric acid. The time required for the tablet to rise to the surface for floating was determined as the Buoyancy lag time and further floating duration of all tablets was determined by visual observation [10].
In Vitro Dissolution Studies
The release rate of repaglinide from floating tablets was determined using United States Pharmacopeia (USP) Dissolution Testing Apparatus 2 (paddle method). The dissolution test was performed using 900 ml of 0.1N hydrochloric acid at 37 ± 0.5oC and 50 rpm. A sample (5 ml) of the solution was withdrawn from the dissolution apparatus hourly and the samples were replaced with fresh dissolution medium. Absorbance of these solutions was measured at 243 nm using a UV/Visible spectrophotometer. The percentage drug release was plotted against time to determine the release profile [5].
Drug Release Kinetics
Drug release data was fitted with different release kinetics models. Zero order, First order, Higuchi model and Koresmeyer-Peppas model [11,12].
Fourier Transform Infrared Spectroscopy (FTIR) studies
The pure drug and formulations were subjected for FTIR analysis. The samples were prepared on KBr-press (Startech Lab, India). The samples were scanned over a range of 4000-400 cm-1 using fourier transformer infrared spectrophotometer. Spectra were analysed for drug polymer interactions.
Differential scanning calorimetry (DSC) studies
The pure drug and optimized formulation were subjected to differential scanning calorimeter equipped with an intra cooler (NETZSCH, Japan.). Indium/zinc standards were used to calibrate the DSC temperature and enthalpy scale. The sample were sealed in aluminum pans and heated at a constant rate 20°C/min over a temperature range of 20-250°C. An inert atmosphere was maintained by purging nitrogen gas at a flow rate of 50 ml/min.
Stability studies
The best formulation was subjected for one month stability study by exposing the tablets in their final packing mode to the temperature 40±2°C and relative humidity 75±5% in programmable environmental test chamber.
Results and Discussion
Physico-chemical evaluation of tablets
The prepared tablets were evaluated for their various physico-chemical properties. The tablets were white, circular in shape and were found to be uniform with respect to thickness (4.60 to 4.67 mm) and hardness (6.3 to 6.8 kg/cm2). The weight variation (1.25-2.12%) and friability (0.25 to 0.42 %) of different batch of tablets were found within acceptable limits. Drug content (93.30 to 98.97 %) was found uniform within the batches of different tablets. The results of physico-chemical evaluation of tablets are given in Table 2.
| Formulati on Code |
Thicknes s+ (mm) |
Hardne ss test+ (kg/cm2) |
Weight variatio n* (%) |
Friabilit y+ (%) |
Drug conten t+ (%) |
| F1 |
4.60 ± 0.02 |
6.3±0.32 |
1.45±0.37 |
0.25 ± 0.12 |
98.97 ± 0.86 |
| F2 |
4.64 ± 0.04 |
6.5 ± 0.33 |
1.35±0.19 |
0.30 ± 0.09 |
98.52 ± 0.67 |
| F3 |
4.62 ±0.01 |
6.6 ±0.34 |
1.30±0.16 |
0.28 ±0.14 |
97.50 ±0.81 |
| F4 |
4.63 ± 0.03 |
6.8 ± 0.46 |
1.25±0.75 |
0.34 ± 0.08 |
93.30 ± 0.66 |
| F5 |
4.67 ± 0.04 |
6.4 ± 0.43 |
2.12±0.16 |
0.42 ± 0.08 |
93.87 ± 0.84 |
All values are expressed as mean ± SD +n=10, *n=20.
Table 2: Physico-chemical evaluation of repaglinide floating tablets.
Swelling study
Investigation of polymer swelling and erosion is a valuable exercise to better understand the mechanism of release and the relative importance of participating parameters. Swelling ratio describes the amount of water that is contained within the hydrogel at equilibrium and is a function of network structure, hydrophilicity and ionization of functional group. Swelling study was performed on all the batches of floating tablet for 24 hrs.All the floating tablets were found intact throughout the period of swelling (24 hrs) in 0.1N hydrochloric acid. The order of swelling index observed with different polymers was Carbopol 934P (F5) > Sodium alginate (F4) >HPMCK100M (F3) >HPMC K15M (F2) >HPMCK4M (F1).Table 3 and Figure 1.
| Time (hrs) |
Swelling Index (%)* |
| F1 |
F2 |
F3 |
F4 |
F5 |
| 0 |
0 |
0 |
0 |
0 |
0 |
| 1 |
40.24 |
45.80 |
49.01 |
53.00 |
47.50 |
| 2 |
61.50 |
63.28 |
67.30 |
65.40 |
63.80 |
| 3 |
78.13 |
79.42 |
80.60 |
79.81 |
75.40 |
| 4 |
92.15 |
94.92 |
97.09 |
95.04 |
90.28 |
| 6 |
104.03 |
106.02 |
109.03 |
110.45 |
112.35 |
| 8 |
120.04 |
122.06 |
128.04 |
129.58 |
132.04 |
| 10 |
138.06 |
140.30 |
145.08 |
148.03 |
150.50 |
| 12 |
152.08 |
153.09 |
158.50 |
160.30 |
165.07 |
| 16 |
178.60 |
180.83 |
183.68 |
185.45 |
194.40 |
| 20 |
190.70 |
194.96 |
198.05 |
200.50 |
210.23 |
| 24 |
205.68 |
209.10 |
215.18 |
220.36 |
235.56 |
*Average of 3 determinations
Table 3: Swelling study of repaglinide floating tablets.
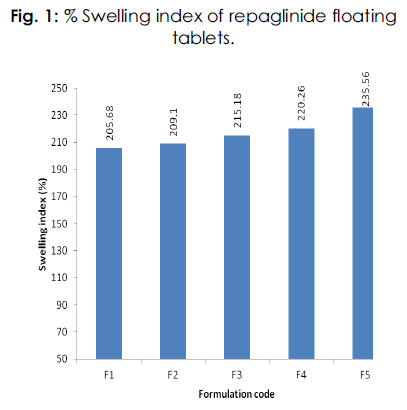
Figure 1: Plasma Na concentration (mmol/L).
Buoyancy lag time and Total floating time
Gastro retentive buoyant tablets need to possess certain characteristics. Therefore experiments were conducted for buoyancy lag time as well as flotation period. Buoyancy lag time indicates how much time a tablet would take, under in vitro simulated conditions to float over the gastric fluid. The tablets were placed in a 250 ml beaker containing 0.1N hydrochloric acid. The time required for the tablet to rise to the surface for floating was determined as the buoyancy lag time.
Sodium bicarbonate induces carbon dioxide generation in the presence of hydrochloric acid. The gas generated is trapped and protected within the gel formed by hydration of the polymer, thus decreasing the density of the tablet below 1 gm/ml and the tablet becomes buoyant. The optimized concentration of sodium bicarbonate was found to be 14% of total tablet weight and it was maintained constant in all the floating tablets prepared. All floating tablets had buoyancy lag time in the range of 33(F4) to 52(F3) sec. The total floating time was found to be in the range of 26(F1and F2) to 34(F4 and F5) hrs, indicating a stable gel layer formation by HPMC, Carbopol 934P and sodium bicarbonate that persists for a longer time. Table 4.
| Formulation Code |
Buoyancy lag time (sec)* |
Total floating time (hrs)* |
t50% (hrs) ** |
t90% (hrs) ** |
| F1 |
45 |
26 |
5.45 ± 0.7 |
15.82 ± 0.2 |
| F2 |
48 |
26 |
5.55 ± 0.4 |
23.50 ± 0.4 |
| F3 |
52 |
30 |
6.07 ± 1.2 |
20.66± 0.3 |
| F4 |
33 |
34 |
5.55 ± 0.5 |
23.52 ± 0.5 |
| F5 |
38 |
34 |
9.54 ± 0.4 |
17.30± 0.1 |
*Average of 3 determinations.
** All values are expressed as mean ± SD. n=3
Table 4: Floating behavior of repaglinide floating tablets.
In vitro release study
Dissolution studies of prepared floating tablets were carried out in 0.1N hydrochloric acid for a period of 24 hrs. The samples were analyzed by UV at 243 nm. The results of in vitro drug release from tablet formulations are presented in Figure 2.
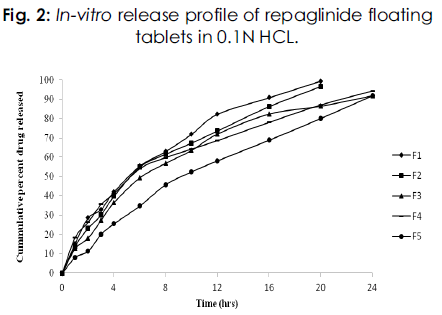
Figure 2: Plasma Na concentration (mmol/L).
In case of formulations F1(HPMC K4M) and F2(HPMC K15M) the tablets released 99.55 and 96.66% of drug in a period of 20 hrs. Formulations F3 (HPMC K100M), F4 (sodium alginate) and F5 (Carbopol 934P) released 91.97, 94.39 and 91.89% of drug respectively in a period of 24 hrs. In all the five formulations, formulation with sodium alginate as polymer released maximum amount of drug in a period of 24 hrs.
Drug Release Kinetics
To investigate the mechanism of drug release from floating tablets, various kinetic models like zero order, first order, Higuchi’s and Korsmeyer- Peppas equations were applied to the in-vitro release data obtained from repaglinide formulations. As observed from Table V, the values of correlation-coefficient (r2) for all the formulations were high enough to evaluate the drug dissolution behaviour. The value of release exponent (n) was found to be a function of polymer used and the physicochemical property of a drug molecule itself.
FT-IR studies
Among the several methods available for establishing physical characteristics of drug formulations, FT-IR spectra and DSC thermograms are frequently used. In the present study the FT-IR When the data was plotted as per zero order kinetics, plots were obtained with lower correlation coefficient values ranging from 0.887- 0.968. First order plots showed high correlation coefficient values ranging from 0.938-0.997. From the observations it was concluded that the order of release was as per First order equation, indicating that the dissolution rate of drug was proportional to the residual drug inside the dosage form. When the drug release data was fitted to Higuchi equation, linear plots were obtained with high correlation coefficient values ranging from 0.977- 0.993. The drug release was proportional to square root of time indicating that the drug release from all the floating tablets was diffusion controlled. The release data obtained were also put in Korsemayer-Peppas model in order to find out n values, which describe the drug release mechanism. The n values of different selected floating tablet formulations were found in the range of 0.623 - 0.830, indicating non-fickian (Anomalous) transport mechanism. Hence the above observations led us to conclude that, all the floating tablets followed diffusion controlled First order kinetics.
spectra of pure drug repaglinide and its formulations F1 to F5 were taken. The characteristics absorption bands of various functional groups and bonds of the drug and its formulations are characterised in the Table 6.
| Formulation Code |
Zero order |
First order |
Higuchi |
Korsmeyer-peppas |
| n |
r2 |
n |
r2 |
n |
r2 |
n |
r2 |
| F1 |
4.752 |
0.920 |
-0.074 |
0.958 |
23.76 |
0.992 |
1.026 |
0.679 |
| F2 |
4.588 |
0.925 |
-0.064 |
0.938 |
22.85 |
0.989 |
1.038 |
0.703 |
| F3 |
3.745 |
0.896 |
-0.044 |
0.997 |
20.82 |
0.982 |
0.990 |
0.734 |
| F4 |
3.467 |
0.887 |
-0.045 |
0.973 |
19.49 |
0.993 |
0.945 |
0.623 |
| F5 |
3.746 |
0.968 |
-0.040 |
0.956 |
19.99 |
0.977 |
1.058 |
0.830 |
Table 5: Kinetic analysis of in-vitro release data of repaglinide floating tablets.
| IR (KBr) cm-1 |
Repaglinide pure drug |
F1 |
F2 |
F3 |
F4 |
F5 |
O-H of CHO and N-H of CONH
stretching |
3308 |
3309 |
3308 |
3308 |
3308 |
3306 |
Aromatic
C-H stretching |
3068 |
3068 |
3068 |
3070 |
3070 |
3070 |
| C=H stretching of CH3and CH2 groups |
2935, 2924, 2804 |
2916 |
2916 |
2916 |
2916 |
2933, 2927 |
| C=O and C=O of CONH |
1685, 1635. |
1685,
1637. |
1685,
1636. |
1685 |
1685,
1636. |
1685,
1636. |
| C=C ring stretching |
1606, 1565, 1500 |
1563 |
1606,
1564. |
1608,
1599 |
1606, 1599 |
1606, 1599 |
| C-H bending of CH3 and CH2 |
1448, 1427,
1382. |
1448, 1382 |
1453, 1382 |
1448,
1382 |
1448, 1382 |
1450 |
| O-H bending |
1255 |
1249 |
1249 |
1246 |
1246 |
1246 |
| C-O-C |
1041 |
1041 |
1041 |
1041 |
1041 |
1041 |
| Substituted phenyl ring. |
862,759 |
867, 759 |
869, 759 |
865, 759 |
857, 759 |
865, 759 |
Table 6: FTIR studies of pure drug repaglinide and its formulations: The IR spectrum of the pure drug and repaglinide formulations showed their characteristic absorption bands and IR peaks (wave numbers, cm-1) in the following IR region.
It is evident from the comparative study of FT-IR spectra of the drug with FT-IR spectra of formulations that there is no appreciable change in the position of characteristic absorption bands. In all the spectra the band positions are within the required range and are in agreement with the literature value. Since there is no change in the characteristics bands in the FT-IR spectra of drug and its formulations, it was concluded that the drug remains in its normal form before and after its formulation. This is only possible in case if the drug has no interaction with polymer and other excipients used during the present study. The above points highlight the fact that there is no interaction of the drug in the formulation with other ingredients. The results of FT-IR studies are shown in Figure 3-8.
Figure 3: Plasma Na concentration (mmol/L).
Figure 4: Plasma Na concentration (mmol/L).
Figure 5: Plasma Na concentration (mmol/L).
Figure 6: Plasma Na concentration (mmol/L).
Figure 7: Plasma Na concentration (mmol/L).
Figure 8: Plasma Na concentration (mmol/L).
Differential Scanning Calorimetry (DSC) study
The DSC thermogram study for drug and its formulations is also utilized for establishing physical characteristics. The DSC thermogram of pure drug gave endothermic peak corresponding to the temperature 134ºC, which indicates its sharp melting point. It was clear form literature survey that the value is in the same reported range, published in different research articles of the drug.
The DSC thermogram of the best formulation even though slightly differs in the nature and appearances show an endothermic peak almost all near to 134ºC.The comparative study of these two thermograms, i.e drug and its best formulation F4 shows the endothermic peak corresponding to the melting point of the drug. Hence it follows that there was no interaction of the drug with polymer and other excipients in best formulation. The results of DSC studies are shown in Figure 9-10.
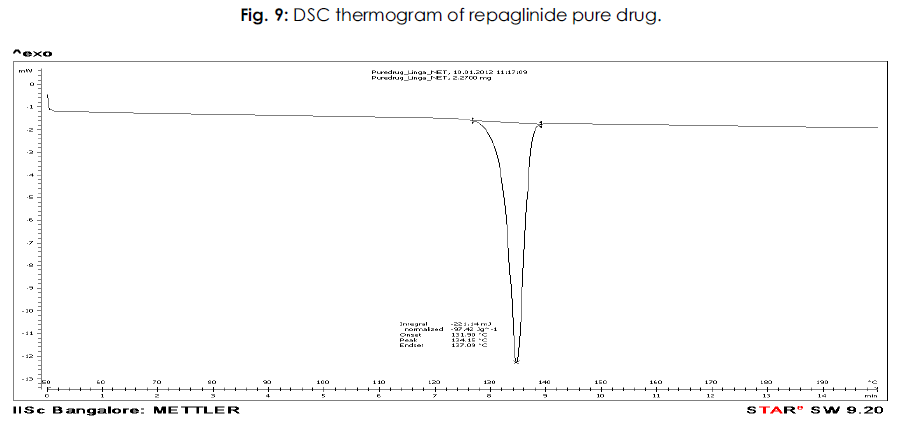
Figure 9: Plasma Na concentration (mmol/L).
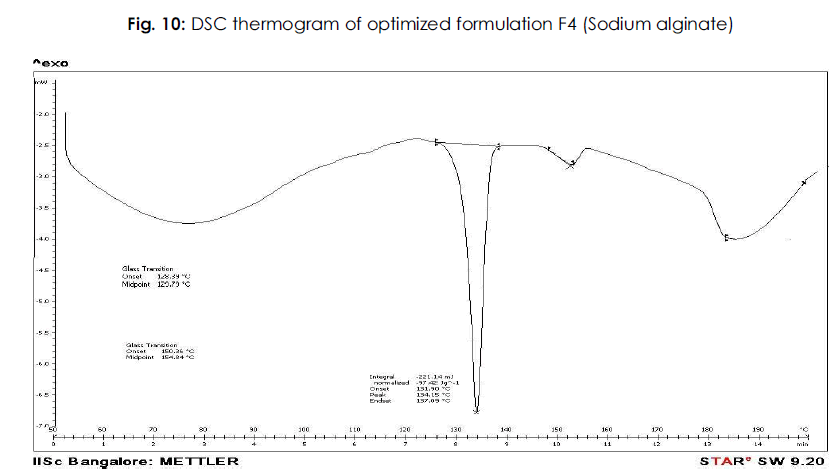
Figure 10: Plasma Na concentration (mmol/L).
Stability studies
The optimized formulation (F4) was stored for a period of 1month at 40±2°C temperature and relative humidity 75±5% in programmable environmental test chamber. The results (not shown) indicated that, the tablets did not show any physical changes during the study period. The drug content was found within limits and no significant variation was observed at the end of one month stability study. T90% and T50% were also not affected during the stability study. In-vitro release studies of optimised formulation showed no significant changes in the drug release. This indicated that, the prepared tablets remained fairly stable during storage conditions.
Conclusion
The polymers used HPMC K4M, K15M and K100M, sodium alginate and Carbopol 934P, showed better drug control over drug release. The effervescent based floating drug delivery containing sodium bicarbonate was a promising approach to achieve in vitro buoyancy. The optimised formulation (F4) gave the best result in terms of required lag time and the floating duration of 24 hrs. The results were encouraging, as longer gastric residence time is an important condition for higher bioavailability of the drugs included in the floating tablets. The dose can be reduced and possible incomplete absorption of the drug can be avoided.
Acknowledgements
Sun pharma.Pvt. Ltd., Mumbai for providing gift sample of Repaglinide. Management, N. E. T. Pharmacy college, Raichur for providing facilities during research.
7288
References
- Milwaukee Wl: Drug evaluations annual, American Medical Association 1993(2).
- Clarke’s Analysis of Drugs and Poisons/monographs/repaglinide. 3rd ed. Pharmaceutical Press; 2005.
- https:// en.wikipededia.org/ wiki/ repaglinide. Accessed on (20-01-2013)
- Sonar GS, Jain DK, More DM. Preparation and in vitro evaluation of bilayer and floating- bioadhesive tablets of rosiglitazone maleate. Asian J Pharm Sci 2007; 2 (4): 161- 69.
- Gupta A. K.: Introduction to pharmaceutics. 2nd Ed. New Delhi: CBS Publications; 1993.
- Lakade SH, Bhalekar MR. Formulation and evaluation of sustained release matrix tablets of anti-anginaldrug, influence of combination of hydrophobic and hydrophilic matrix former. Research J Pharm and Tech 2008; 1(4): 410 -13.
- Banker G. S. and Anderson N. R.: Tablets In: Lachman N, Liberman HA, Kanig JL, editors. The theory and practice of industrial pharmacy. 3rd ed. Bombay: Varghese Publication House; 1987: 286-300
- Nerurkar J, Jun HW, Prince JC, Park MO. Controlled release matrix tablets of ibuprofen using cellulose ethers and carrageenans: effect of formulation factors on dissolution rate. Eur J Pharm Biopharm 2005; 61: 56 - 68.
- Puneeth KP, Kavitha K, Tamizh MT. Development and evaluation of rosiglitazone maleate floating tablets. Int J Appl Pharm 2010; 2(2): 6 -10.
- Reza MS, Quadir MA, Haider SS. Comparative evaluation of plastics, hydrophilic polymers as matrices for controlled-release drug delivery. J Pharm Sci 2003; 6(2): 282 -91.


















