Key words
|
| |
| Aceclofenac, Matrix tablets, Sustained release, Wet Granulation, Hydroxy Propyl Methyl Cellulose (HPMC), F-2 (similarity factor). |
| |
Introduction
|
| |
| Non-steroidal anti-inflammatory drugs (NSAIDs) are considered to be the first-line drugs in the symptomatic treatment of rheumatoid arthritis, osteoarthritis and ankylosingspondylitis, aceclofenac is one of them[1]. It is a newer derivative of diclofenac with low gastrointestinal complications. The short biological half-life (about 4 hr) and dosing frequency more than one per day make aceclofenac an ideal candidate for sustained release. To reduce the frequency of administration and to improve patient compliance, a once-daily sustained release formulation of aceclofenac is desirable. Matrix tablets composed of drug and polymer as release retarding material offer the simplest approach in designing a sustained release system. The present study aims to develop sustained release matrix tablets using hydrophilic matrix materials[2], such as Hydroxy Propyl Methyl Cellulose (HPMC) of various grades as polymer with drug in varying proportions by wet granulation method. |
| |
Materials and Methods
|
| |
| Aceclofenac B.P., HPMC K4M, HPMC K15M, Poly vinyl pyrrolidone k30, Isopropyl alcohol, Magnesium stearate, Microcrystalline cellulose, Methanol, Tween 80 were obtained as gift samples from Swiss Medicare Pvt. Ltd, Sri Ganganagar (RAJ.) respectively. All other chemicals used were of analytical grade. |
| |
| Physical evaluation of powders: The powders were evaluated for angle of repose, bulk density, tapped density, compressibility index, Hausner ratio, and drug content etc.[3] |
| |
| Bulk density: An accurately weighed quantity of the powder (W) was carefully poured into the graduated cylinder and the volume (Vo) was measured then the graduated cylinder was closed with lid, set into the density determination apparatus (Bulk density apparatus, electro lab, Mumbai).[7] The density apparatus was set for 500 taps and after that, the volume (Vf) was measured and continued operation till the two consecutive readings were equal. The bulk density and tapped density were calculated using the following formulas: |
| |
| Bulk density = W/Vo |
| |
| Tapped density = W/Vf |
| |
| Where, |
| |
| Vo = Initial Volume |
| |
| Vf = Final Volume |
| |
| Compressibility index & Hausner Ratio: The compressibility index and Hausner ratio may be calculated using measured values for bulk density (Ebulk) and tapped density (Etapped)as follows : |
| |
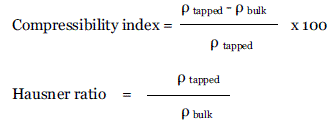 |
| |
| Angle of Repose: Angle of repose is defined as the maximum angle possible between the surface of a pile of the powder and the horizontal plane. Where, |
| |
| θ = tan-1 h/r |
| |
| h = height of pile |
| |
| r = radius of the base of the pile |
| |
| θ = angle of repose |
| |
| Loss on drying: Determination of loss on drying of granules is important drying time during granulation was optimized depending LOD value. LOD of each batches were tested at 105°C for 2.5 minutes by using “Sartorius” electronic LOD apparatus. |
| |
| Drug content: An accurately weighed amount of powdered aceclofenac (100 mg) was extracted with water and the solution was filtered through 0.45-μ membrane filter paper. The absorbance was measured at 275 nm after suitable dilution. |
| |
| Preparation of tablets: The tablet was prepared by using Wet Granulation Method. Weight accurately Drug + HPMC K15M + PVP K-30 and microcrystalline cellulose passes through 40 no. sieves and mix properly for 3-5 minutes in a steel tub. Prepare binder solution by dispersing PVP K30 in isopropyl alcohol. Granulation of above mixture is done by prepared binder solution by kneading up to granulation end point is obtained (Dough mass). Pass the dough mass through 12 mesh and keep it in a tray dryer for drying and finally keep the loss on drying (LOD) up to 2-3 %. Remove the dried granules from oven and pass through 20 mesh sieve to get optimum size granules. Lubrication is done by using magnesium stearate and passed through 60 mesh of the granules for 3 to 4 min. in a steel tub and then in polybag. Compression is done by using 16 station single rotary tablet punching machine (made CADMACH) by using 9.6 mm round, biconcave, both side plane punch. |
| |
| Physicochemical characterization of tablets: The thickness and diameter of the tablets were determined using digital vernier callipers. The hardness of the tablets was determined by using Monsanto hardness tester. The friability of the tablets was determined using Elector lab Friabilator. Weight variation test of the tablets was carried out as per the official method[5]. For determining the drug content, three tablets were crushed and powder containing 200 mg of aceclofenac was dissolved in 100 ml of methanol. The solution was passed through filter and analyzed by UV spectrophotometer at 275 nm after sufficient dilution with phosphate buffer (pH 6.8). |
| |
| Dissolution studies: The release study was carried out for 24 hours using USP 22 paddle type dissolution apparatus in buffer (pH 6.8) at 75 rpms maintaining temperature. A 10ml samples were collected from each vessel at 2, 4, 8, 12, 16 and 24 hour and analyzed by UV spectrophotometer at 274 nm. The withdrawn sample was immediately replaced by equal volume of fresh buffer. |
| |
| Stability studies: After determining drug content, the tablets were charged for the accelerated stability studies according to ICH guidelines (40 ± 2°C and 75 ± 5% RH) for a period of 2 months in stability chambers. The samples were taken out at 30 and 60 days and evaluated for the drug content, dissolution, related substances and physical parameters like hardness and friability[4]. |
| |
Results and Discussion
|
| |
| Micromeritic properties: The results of micromeritic properties are presented in Table 2.The sustained release granules and tablets of Aceclofenac were prepared by wet granulation method, the granules were evaluated for determination of bulk density and tapped density, Compressibility index, Hausner Ratio, Loss on drying and Angle of repose. The tablets were evaluated for weight variation, drug content, friability, hardness, and thickness for all batches (T-1 – T-10)[5]. |
| |
| Evaluation of prepared tablets: The results of physicochemical evaluation of tablets are given in Table 3. The tablets of different batches were found uniform with respect to thickness (4.43-4.72 mm) and hardness (4 to 6 kg/cm2). The friability (%) and weight variation of different batches of tablets were found within the prescribed limits (friability: 0.42 to 0.67%; deviation of weight variation test: 1.97 to 3.16%). Good and uniform drug content (>96%) was observed within the batches of different tablet formulations. |
| |
| Hence, the tablets containing drug, HPMC, MCC and magnesium stearate could be prepared satisfactorily by wet granulation method. The release of Aceclofenac from sustained release tablet of various formulations varied according to the ratio and degree of the polymer. In case of tablet of T1 containing drug & HPMCK15M (quantity in mg). 200: 15 the release profile was showing the release 101.64%. In case of tablets of T2 containing drug and HPMC K15M & HPMC K4M (in mg) 200:10:20 it was showing 100.26% release in 24 hours. In case of tablets of T3 containing drug polymer’s (HPMC K15M, HPMC K4M in mg) 200 : 15 : 15 prepare to be seen in the effect of combination of polymers in release of drug but it was showing release 102.45% up to 24 hour. In case of tablets T4 containing drug and HPMC K15M & HPMC K4M (in mg) 200: 10: 10 the release profile was showing drug release 100.68% .In case of tablets of T5 containing drug and HPMC K 4M & HPMC K15M PVP K30 (in mg) 200: 10: 10:10 prepared the tablets was showing drug release 100.86%. |
| |
| In case of tablets of T6 containing drug and HPMC K15M (in Mg) 200:5 was showing drug release 101.54% in 24 hour profile. In case of tablets T7 containing drug, HPMC K4M & HPMC K15m (in mg) 200:10:10 the release profile was showing drug release 101.67%. In case of Tablets T8 containing drug HPMC K15M (in mg) 200:23. The release profile was showing drug release with in 24 hours. With very slower release than all formulations containing % drug release 99.86. |
| |
| In case of tablets T9 containing drug, HPMC K4M (in mg) 200:20 the release profile was showing drug release 102.98%. In case of tablets T10 containing drug, HPMC K4M (in mg) 200:25 the release profile was showing drug release 100.26%. The drug release profile of marketed product was showing drug release 99.07 %. |
| |
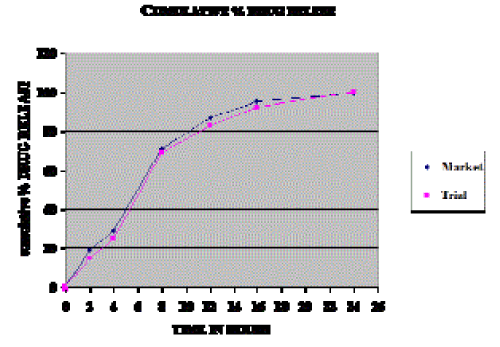
| Comparative Study: The results indicated that T8 released the drug in a manner which is almost similar to the marketed tablet. Hence T8 can be considered as better formulation among the prepared sustained release tablets. |
| |
| F1 & F2 Factor: The results indicated that T8 released the drug in a manner which is almost similar to the marketed tablet. Hence T8 can be considered as better formulation among the prepared sustained release tablets. The similarity in the release profiles of marketed tablet and formulation T8 was compared by making use of “Model independent approach”. A simple model independent approach uses a difference factor (f1) and a similarity factor (f2) to compare dissolution profiles (https://www.fda.ov/cder/guidance). For T8 formulation, when compared with marketed tablet, f1 and f2 values were found to be 4.68 and 73.90 respectively, indicating a good equivalence between these formulations. |
| |
| Stability studies: The results of accelerated stability studies, carried out according to ICH guidelines, indicated that the tablets did not show any physical changes (colour change, friability and hardness) during the study period and the drug content was found above 99% at the end of 60 days (0 day: 99.24; 60 days: 99.15). This indicates that T8 tablet is fairly stable at accelerated storage condition. However real time stability studies for a period of 02 months are required to establish the stability of developed product. |
| |
Conclusion
|
| |
| In the present study, the formulation and production technology of aceclofenac 200 mg hydrophilic matrix tablets have been developed, which produced S.R formulation with good physical characteristics, predictable and reproducible drug release profiles similar to the marketed reference product. This study demonstrated that HPMC K15M provides reliable sustained release matrix formulation recommendations for high dose of aceclofenac. |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
Figure 2 |
|
| |