Keywords
5CG-Desiccator; IC50 and IC90 determination; Plant extracts; Trypanosoma brucei brucei
Introduction
Several species of trypanosomes are the causative agents of African Trypanosomiases, a disease responsible for death and morbidity of man and his domestic animals in the tropical Africa. According to World Health Organization [1], approximately 300,000 to 500,000 people are affected by Human African Trypanosomiasis (HAT) with an estimated $ 4.5 billion annual economic losses accrued to animal trypanosomiasis. To date no effective vaccine, against any species of African trypanosomes, is in commercial circulation [2] and the trypanocides in use are faced with several challenges including high cost of purchase, toxic side effects associated with intake and the growing incidence of drug resistance developed by various species of the parasite [3].
In an attempt to source for easily available and safer agents, different synthetic and plant-derived compounds are being screened for their antitrypanosomal potentials [3-6]. In vitro screening procedures of substances against trypanosomes require the parasite to be cultured axenically. Today, there are several long term culture techniques for the cultivation of various species of trypanosomes which have been adapted and used in many research laboratories [4-9]. The practice involves culturing organisms under 5% CO2 concentration in a CO2 incubator, equipment that is expensive and not readily available in most laboratories in the developing countries of the world. In addition, the equipment relies on steady supply of electricity which is usually a problem to researchers in such countries. As a result of this challenge, scientists in developing countries had employed in vitro cultivation time for as short as an hour while screening extracts for their antitrypanocidal potentials [5,10,11]. Even though some measure of success was recorded, the equipment had the limitation of using only drop in parasite motility as index for parasite mortality. Even though motility may reflect parasite viability, sometimes, immotile organisms could retain their infectivity and in such case, motility could give misleading in vitro results, thereby questioning the reliability of data generated. Again, since, no sufficient time was given for parasite-extract interaction, it was impossible, by this approach; to determine the IC50 or IC90 values of the plant extracts and so preclude any comparative study of screened antitrypanocidal principles. Review of literature revealed that studies on the African medicinal plants against trypanosomes are few [12].
Recent effort to source for alternative field-adapted culture system to CO2-incubator utilized a glass desiccator gassed with laboratory prepared CO2 [13]. This study will explore the possibility of using the 5CG desiccator for in vitro microassessment of the trypanocidal potentials of ethanolic extracts of Cymbopogon spp, M. oleifera, V. amygdalina and A. sativum, medicinal plants indigenous to northern Nigeria against Trypanosoma brucei brucei.
Materials and Methods
Plants collection and identification
Fresh leaves samples of ten plants (Alium sativum (AS), Aloe vera (AV), Azadirachta indica (AI), Carica papaya (CP), Cymbopogon spp (CS), Eucalyptus spp (ES), Khaya senegalensis, Moringa oleifera (MO), Mitracarpus scaber (MS) and Vernonia amygdalina) were collected within Kaduna town and taken to the Department of Biological Sciences, Kaduna State University, and Kaduna Nigeria for identification.
Experimental animals
Rats and mice raised at the animal colony of the Department of Biochemistry, Kaduna State University, Nigeria, were used for parasite maintenance and as source of parasites for the in vitro culture experiments. All animals were kept in accordance with accepted standards.
Parasite
The parasite, T. b. brucei, was obtained from the Nigerian Institute for Trypanosomiasis Research (NITR), Kaduna and was maintained by serial passage in mice and rats.
Chemical/reagents
Diethylaminoethyl cellulose (DE52) and Tris base were purchased from Sigma Chem. Co. (USA). Amino acids, Sodium Chloride, Sodium Dihydrogen Phosphate (NaH2PO4.2H2O), Disodium Hydrogen Phosphate (Na2HPO412H2O), Glucose, Antibiotics (Ampicillin, Streptomycin), Ethylenediamine Tetraacetic acid (EDTA), Chloroform, Glycerol, Giemsa powder, Calcium Carbonate (Ca2CO3) were all of analytical grade.
Preparation of plants extracts
Leafy part of each Plant was collected fresh and chopped into pieces using a sterile knife. Exactly 100 g of the chopped plant was transferred into the blender, and 250 ml of ethanol was added before blending. The blended plant material was transferred into a clean 500 ml glass bottle and allowed to extract by macerations for 12 hrs. Thereafter, it was filtered with cheese cloth and then with double layers of Whatman filters paper. The filtrate was collected and concentrated using rotary evaporator. The concentrate was transferred into a cleaned weighed crucible and placed in a water bath set at 45°C until the residual solvent is fully removed. The dried extract was then stored in a refrigerator at 4°C until required.
Preparation of goat serum
Fresh blood sample was collected from young apparently healthy goat into sterile 10 ml centrifuge tubes and centrifuged at 3000 rpm for 10 minutes. The serum was heatinactivated at 55°C for 1 hour and stored in aliquots of 10 mls in 10 ml centrifuge tubes at -20°C.
Constitution of the culture medium for in vitro studies
The culture medium, Eagle minimum essential medium, was manually prepared using composition defined by Eagle [14]. The amino acids, vitamins, inorganic salts, phenol red and the antibiotic filtered components of the medium were separately prepared and stored in aliquots of 1 ml at -20°C. Prior to experiments, aliquots were taken and reconstituted to the required concentrations of the Eagle’s medium. Goat serum (prepared from freshly collected blood) and glucose were added to the medium to achieve a 20% and 1% concentration respectively as previously described [15].
In vivo growth and Isolation of T. b. brucei from infected animals
At log phase parasitaemia, infected rats (weighing between 100-150 g) were sacrificed after chloroform anesthesia and through cardiac puncture, blood were collected into syringes containing 1% (w/v) EDTA solution in 100 mM phosphate buffer saline (0.2 ml of EDTA: 2 ml of blood). Collected blood were pooled into 10 ml centrifuge tubes, concentrated (3000 rpm for 10 minutes) and the parasites which aggregate a white cloudy buffy coat were carefully picked using pasteur pipette into a sterile microfuge tube containing phosphate saline glucose (100 mM PSG, pH 7.2, 0.2% (w/v) Glucose) and then subsequently freed of all erythrocytes and leucocytes contaminants via passage through ion exchange chromatographic column as described by Lanham and Godfrey [16]. Briefly, DE52 cellulose matrix was dissolved in phosphate buffer saline glucose and loaded on a 20 ml syringe column whose outlet was blocked with little cotton wool. The buffy coat parasites were applied on the column pre-equilibrated with phosphate buffer saline glucose (pH 8.0). By this procedure, the residual red cells were attached to the matrix while the parasites run down the column as a thick white suspension. The column is continually washed until eluent is transparent again. The suspension was then centrifuged at 2500 rpm for 10 minutes, the supernatant was discarded and the parasites pellet washed thrice with PBS (pH 7.2) before they were finally re-suspended in the cultivation medium and the parasite density determined using haemocytometer.
Counting of parasite in suspension and culture medium
Parasite densities in the culture media were determined using New Improved Neubauer counting chamber [13] described previously [15].
In vitro micro-assessment studies
Stock extract solutions were prepared by dissolving 1 mg of each plant extract with 1 ml of the culture medium containing 1% D-glucose, buffered with 25 mM Hepes to pH 7.4 and supplemented with 20% heat-inactivated goat serum. One hundred micro liters of this stock extract-medium and the various dilutions made from it were dispensed in triplicates into wells of 96 wells micro titer plate producing a range of concentrations from 1000 g/ml to 7.8 g /ml. To each well, 20 l of the parasite suspension (containing approximately 10000 parasites and producing parasite density of 8 × 104 parasite/ml) was added and the plate was then covered and put into a glass desiccator and the carbon dioxide gas (in amount that is equivalent to 5% of the capacity of the desiccator) was immediately released into the desiccator at 5% concentration (from where the 5 CG acronym was derived). The details of the preparation of the carbon dioxide have been described elsewhere [15]. The effective concentrations produced were from 995 to 6.5 g/ml. To establish the extent of extracts activities, parasite in the micro litre plate were incubated at 27°C for 24 hrs in the presence of the extracts and the parasite densities at the end of the incubation period were determined using a haemocytometer and compared with densities obtained from Negative control (parasite cultured in 100 l of the medium without extract or standard drug) and Standard drug control (parasite cultured in 100 l of 1000 g/ml of Diminazine Aceturate) wells. For each extract or drug the concentration which inhibit parasite population by 50% (IC50) and 90% (IC90) were determined by interpolation method of Hills as explained by Huber and Koella [17] and used by Scory and Steverding [9].
Results
The mean IC50 and IC90 values of all the extracts were determined using two separate graphical methods. In the first approach the parasite population (%) was plotted on the Yaxis directly against extract concentration on the X-axis (Figure 1). From this graph, it was difficult to extrapolate IC50 values for concentration less than 50 mg/ml due to clustering of the curves around this region. The IC90 values determination, however, did not pose any challenge since the use of logarithmic parameter on the X-axis gave rise to well-spacedout plots (Figure 2) around portions leading to IC50/ IC90.
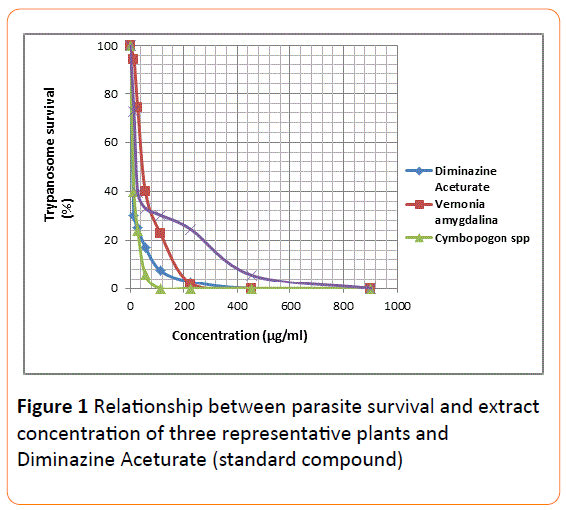
Figure 1: Relationship between parasite survival and extract concentration of three representative plants and Diminazine Aceturate (standard compound).
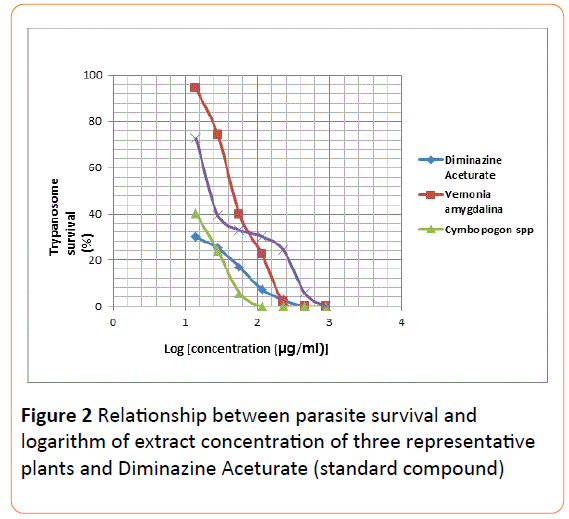
Figure 2: Relationship between parasite survival and logarithm of extract concentration of three representative plants and Diminazine Aceturate (standard compound).
Discussion
The IC50 and IC90 values of ethanol extracts of ten medicinal plants were evaluated using a 5CG-Desiccator culture technique. This is of great significance because despite reports on the antitrypanosomal activities of extracts from local plants, the IC50 values of most of them remain unknown [12], providing limited opportunity for comparative analysis of their trypanocidal potentials.
The 5CG-Desiccator culture technique earlier developed in this laboratory and reported [15] was used successfully to culture the trypanosomes.
It proved effective for the screening of all the extracts tested. The trypanosomes remained alive and active in vitro for the 24 hr duration required for the micro-assessment of extracts' activities, thus confirming the previous observation [15].
Extrapolation of the IC50 values from the two graphical methods employed (Figures 1 and 2) using data of some representative extracts was difficult. This difficulty was attributed to the absence of extract dilutions below 14.2 g/ml. There is a need to have lower concentrations (as low as 0.1 g/ml) as part of the series of dilutions employed, in order to have values less than 1 on the Logarithm (concentration) scale.
Table 1 presents the comparative results obtained for IC50 and IC90 of all the ethanolic extracts using the two methods earlier mentioned. Mean values obtained were fairly consistent irrespective of the method adopted. The IC90 values were identical for both methods of extrapolation. Higher values appeared to be associated more with the logarithmic plot than with the direct concentration plot.
| Plant /Drug |
Method-I Concentration vs % Survival |
Method-II Log Concentration vs % Survival |
| IC50 values (μg/ml) |
IC90 Values (μg/ml) |
IC50 values (μg/ml) |
IC90 values (μg/ml) |
| Diminazine Aceturate |
DE† |
90 |
DE |
89.13 |
| Azadirachta indica |
300 |
420 |
316.23 |
416.87 |
| Khaya senegalensis |
50 |
680 |
50.12 |
602.56 |
| Carica papaya |
DE |
820 |
ND |
891.25 |
| Cymbopogon spp |
12 |
40 |
ND |
41.68 |
| Eucalyptus spp |
DE |
370 |
21.88 |
380.19 |
| Moringa oleifera |
85 |
190 |
95.4 |
199.52 |
| Mitracarpus scaber |
>1000 |
>1000 |
>1000 |
>1000 |
| Vernonia amygdalina |
40 |
160 |
41.68 |
158.49 |
| Allium sativum |
DE |
140 |
ND |
151.35 |
| Aloe vera |
100 |
540 |
100 |
562.34 |
| † Values are either difficult to estimate (DE) by the method employed or not determined (ND) at all. |
Table 1: The IC50 and IC90 Values of the ethanolic extracts extrapolated using two approaches.
The results showed that four of the plant extracts (Cymbopogon spp, M. oleifera, V. amygdalina and A. sativum) had IC90 values less than 200 μg/ml, five (A. indica, K. senegalensis, C. papaya, Eucalyptus spp and A. vera) had values between 200 μg/ml and 1000 μg/ml and one (Mitracarpus scaber) had value greater than 1000 μg/ml.
Several incubation periods are used to determine the IC50 values of various compounds [4-6,18,19]. Most importantly, IC50 values are generally been used to compare different biological responses under the effect of different drugs/ extracts. Results from the present study were obtained using 24hr incubation period since the duration is sufficient to allow for several rounds of parasite growth cycle and parasite-drug interaction [15].
It seems, however, that extrapolation from graph of (parasite survival vs logarithm of the concentration) will be much easier as the lines were more stretched out and better spaced apart (Figures 1 and 2).
For the determination of IC90 values both graphical approaches were effective (Table 1). Therefore, the comparative trypanocidal analysis of the studied extracts was based on the IC90 results presented in Table 1. Extract of Cymbopogon spp was adjudged most trypanocidal (having IC90 value of about 42 g/ml) while Mitracarpus scaber extract was considered least trypanocidal (with IC90 value above 1000 g/ml).
A number of workers have studied both the in vitro and in vivo antitrypanosomal properties of most of the plants under investigation [3,20,21] with varying outcome. Unfortunately, standard in vitro trypanocidal studies (involving the determination of IC50) were not carried out for most of these plants by local researchers in the tropical countries were the plants grow in their natural habitats. Inability to culture trypanosomes for up to 24 hrs was a major hindrance requiring carbon dioxide which the 5CG-desiccator culture has partly addressed.
As a way of optimizing the utility of this culture method, we recommend that the IC50 values should be determined in phases: the first phase should be devoted to testing concentration from 0.01 to 100 g/ml while subsequent phase (from 100 to 1000 g/ml or less than 0.01 g/ml) should be left for extracts whose IC50 values are visibly well above 100 g/ml or well below 0.01 g/ml. The phase two experiment should only be carried out if the IC50 could not be found by the phase one experiment.
Funding
The study was collectively funded by the authors.
Competing and Conflicting Interests
The authors do not have any conflicting interest whatsoever.
Ethical Approval
The study was approved by the Institutional Research Ethics Committee.
Acknowledgments
The authors wish to thank the Management of Nigerian Institute for Trypanosomiasis Research for providing the test parasite used in the study and the Vice Chancellor, Kaduna State University, Nigeria, Prof. B.W. Qurix for permission to publish.
9298
References
- Moussiaux NA, Buscher P, Desmecht D (2009) Host-parasite interactions in trypanosomiasis: on the way to an antidisease strategy. Infect Immun 77: 1276-1284.
- Nok AJ, Esievo KAN, Ishaya H, Samuel, Arowosafe PC, et al. (1993) Trypanocidal potential of Azadirachtaindica: in vivo activity of leaf extract against Trypanosomabruceibrucei. Journal of Clinical Biochemistry and Nutrition 15:113-118.
- Adewunmi CO, Agbedahunsi JM, Adebajo AC, Aladesanmi AJ, Murphy N, et al. (2001) Ethno-veterinary medicine: screening of Nigerian medicinal plants for trypanocidal properties. J Ethnopharmacol 77: 19-24.
- Kelly JM, Quack G, Miles MM (2001) In Vitro and In Vivo Activities of Aminoadamantane and Aminoalkylcyclohexane Derivatives against Trypanosomabrucei. Antimicrobial Agents and Chemotherapy 45: 1360-1366.
- Shuaibu MN, Kanbara H, Yanagi T, Ichinose A, Ameh DA, et al. (2003) In vitro trypanocidal activity of dibutyltin dichloride and its fatty acid derivatives. Parasitol Res 91: 5-11.
- Hirumi H, Doyle JJ, Hirumi K (1977) African trypanosomes: cultivation of animal-infective Trypanosomabrucei in vitro. Science 196: 992-994.
- Baltz T, Baltz D, Giroud C, Crockett J (1985) Cultivation in a semi-defined medium of animal infective forms of Trypanosomabrucei, T equiperdum, T.evansi, T rhodesiense and T gambiense. EMBO J 4: 1273-1277.
- Scory S, Steverding D (1997) Differential toxicity of ricin and diphtheria toxin for bloodstream forms of Trypanosomabrucei. Molecular and Biochemical Parasitology 90:289-295.
- Atawodi SE, Bulus T, Ibrahim S, Ameh DA, Nok AJ, et al. (2003) In vitro trypanocidal effect of methanolic extract of some Nigerian savannah plants. African Journal of Biotechnology 2: 317-321.
- Bulus T, Atawodi SE, Mamman M (2008) In vitro Antitrypanosomal Activity and Phytochemical Screening of Aqueous and Methanol Extracts of Terminaliaavicennioides. Nigerian Journal of Biochemistry and Molecular Biology 23: 7-11.
- Ibrahim MA, Mohammed A, Isah MB, Aliyu AB (2014) Anti-trypanosomal activity of African medicinal plants:a review update. J Ethnopharmacol 154: 26-54.
- Turgeon ML (2007) Clinical laboratory science the basics and routine techniques. Mosby-Elsevier pp: 263-275.
- Eagle H (1955) The specific amino acid requirements of a human carcinoma cell (Stain HeLa) in tissue culture. J Exp Med 102: 37-48.
- Bulus T, Ahmed AB, Sani ZM, Sa’id HH, Samdi SM (2012) Adaptation of a 5CG-Desiccator for in vitro cultivation of Trypanosomabruceibrucei. Archives of Clinical Microbiology 3: 4.
- Lanham SM, Godfrey DG (1970) Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. ExpParasitol 28: 521-534.
- Huber W, Koella JC (1993) A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop 55: 257-261.
- Kaminsky R, Zweygarth E (1989) Feeder Layer-Free In Vitro Assay for Screening Antitrypanosomal Compounds against Trypanosomabruceibrucei and T. b. evansi. Antimicrobial Agents and Chemotherapy 33: 881-885.
- Raz B, Iten M, Buhler GY, Kaminsky R, Brun R (1997) The Alamar Blue assay to determine drug sensitivity of African trypanosomes (T.b. rhodesiense and T.b. gambiense) in vitro. Acta Trop 68: 139-147.
- Atawodi S (2005) Comparative in vitro trypanocidal activities of petroleum ether, chloroform, methanol and aqueous extract of some Nigerian Savannah Plants. African Journal of Biotechnology 4: 177-182.
- Atawodi S, Shehu H (2010) Antitrypanosomal property of some extracts of different parts of Moringaoleifera, Lam. Electronic Journal of Biology 6: 19-23.







