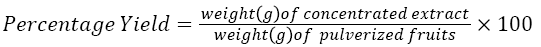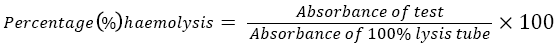Keywords
Osmotic fragility; Red cells; Albino rats; Haemolysis; Indomethacin
Introduction
Tetrapleura tetraptera (TTE) is a Fabaceae. It is regarded as “Aridan” or “Aidan” among the Yorubas in Nigeria; “Abogolo” by the Igalas of North- Central Nigeria; “Dawo” by the Hausas and also Uhikiriho/Oshorisho by the Igbos; but Ushagisha/ Ushagusha by the Nsukkas of South- Eastern Nigerian [1,2] and in Eha-amufu, it is known as Oszagusza.
It is mainly seen in the lowland forests of many tropical African countries and often grows to a height of 20-25 meters with a girth of 1.5-3.0 meters and fairly smooth and thick bark. The leaves are sessile, glabrous or minutely hairy with a common stalk. It is reported that the flowers which are pinkish-cream in colour usually change orange with time while the fruit is very persistent and hangs at the end of branches on stout stalks 25 cm long and consists of fleshy pulp and small, brownish-black seeds, a characteristic fragrant and pungent aromatic odour [2,3]. In Ghana, the tree is usually deciduous in December and starts to flowers between late February and early April and its indehiscent pods are ripe from September to December [3].
Aim
The study was done to determine the effect of Tetrapleura tetraptera methanol extract fruit osmotic fragility (Membrane stability) of erythrocytes, platelet aggregation, and phospholipase A2 activity.
Materials and Methods
Materials
Collection of Tetrapleura tetraptera fruit: The dry fruits of Tetrapleura tetraptera were obtained from Eke market, Eha- Amufu and identified by Dr. Garuba Omosun at the Department of Plant Science and Biotechnology, Michael Okpara University of Agriculture, Umudike. The TTE fruits were washed with distilled water and chopped to expose the seeds in order to remove seeds and the fruits only pulverized. Fifty 50g of the powdered material were placed into the extraction chamber of the soxhlet extractor and extraction was done using methanol as solvent and extraction temperature was maintained at 64.7°C for 48 hours.
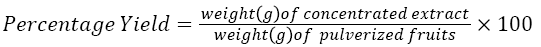
Animals: Twenty albino rats of different gender measuring 85-130 g were obtained from the Animal Production Unit of the College of Veterinary Medicine, Michael Okpara University of Agriculture, Umudike. They were allowed to acclimatize to laboratory conditions for 2 weeks, under best conditions with 12 hours light and dark cycle. The animals were given standard feed and water ad libitum but starved for 12 hours before the beginning of practical. The practical was conducted in compliance with NIH guidelines for care and use of laboratory animals by Akah et al. [4].
The research was done in the physiology laboratory of the department of physiology and pharmacology, College of Veterinary Medicine of the University.
Methods
Design of the experiment: A total of 20 albino rats of different gender measuring 85-130 g weight were randomly grouped into 4 groups of five rats each. The groups were as follows:
• Group 1 (Negative control): Rats fed with normal diet and water ad libitum only for fourteen days (14).
• Group 2 (Positive control): Rats fed with normal diet and water ad libitum for fourteen days (14) but given 10 mg/kg b. w Aspirin (Standard drug) at 1 hr. before induction of hind paw oedema (using 0.3 ml/kg b. w fresh egg albumin).
• Group 3: Rats fed with normal diet and water ad libitum and 200 mg/kg b. w of Tetrapleura tetraptera fruit extract (TTE) for fourteen days (14) and also at 1 hr. before induction of hind paw oedema (using 0.3 ml/kg b. w fresh egg albumin).
• Group 4: Rats fed with normal diet and water ad libitum also treated with 400 mg/kg b. w of Tetrapleura tetraptera fruit extract (TTE) for fourteen days (14) and also at 1 hr. before induction of hind paw oedema (using 0.3 ml/kg b. w fresh egg albumin).
At the end of the experiment, the rats were sacrificed and blood samples collected by cardiac puncture using sterile syringes and needles. The blood samples were divided into two portions. One portion (about 2 ml) was transferred into sample bottles containing EDTA (anticoagulant) and used for the assessment of haematological parameters. The other portion of blood was collected in sterile plain tubes without anticoagulant and allowed to clot for 40 minutes. The blood samples were spun at 5,000 rpm for 10 minutes using centrifuge. The sera were collected, transferred to sterile bottles and kept for analysis in the refrigerator.
Platelet aggregatory activity: The effect of TTE extract on platelet aggregation was determined according to Nwodo [5] which was modified by Born and Cross [6]. Venous blood was collected from apparently healthy subject who had not taken drug for at least two weeks and 1ml was added into plastic tubes containing 0.1 ml volume 3.8% trisodium citrate and centrifuged at 5,000 xg for 20 min at room temperature. The supernatants were obtained as platelet rich plasma (PRP). Reaction medium (2.5 ml) containing 2.0 ml normal saline and 0.5 ml of PRP was used as the control.
Changes in absorption of the PRP were obtained at room temperature as follows: PRP sample of 0.5 ml were added into plastic tubes containing saline and CaCl2 as the inducer of aggregation. The mixtures were progressively turned with a magnetic stirrer, as the alterations in absorption were obtained at interval of 2 min, 4 min, 6 min and 8 min. This was done with graded concentrations of the extract. At the 8th min, there was maximum aggregation.
Assay of phospholipase A2 activity: The phospholipase A2 activity was measured by the modified method of Roelofsen et al. [7] using enzyme gotten from Bacillus cereus. The assay medium contained 4 mm CaCl2 to increase the activity of the enzyme. The assay medium (2.5 ml) containing normal saline, calcium chloride (0.1 ml), RBCs (0.5 ml) without enzyme was used as the negative control, whereas the medium (2.5 ml) containing normal saline, calcium chloride (0.1 ml), RBCs (0.5 ml) and enzyme (0.2 ml) was regarded as the positive control. In tests were initiated by the addition of 0.2 ml enzyme preparation. The tubes were left undisturbed at room temperature (37°C) for 1 hr., then incubated and later centrifuged at 3,000 xg for 10 min to terminate the reaction. The absorptions of the supernatants were read against that of the blank at 418 nm. This was done in the presence and absence of graded concentrations of the extract.
Determination of osmotic fragility (membrane stabilization)
Membrane stabilization was carried out using the method described by Ochei and Kolhatkar, [8].
Reagents: The solution of sodium chloride stock osmotically equivalent to 10% is prepared as follows:
• Sodium chloride-90 g
• Disodium hydrogen phosphate-13.65 g
• Sodium dihydrogen phosphate-2.34 g
• Distilled water-1 litre
Specimen: Heparinised venous blood was used. Oxalated, citrated or EDTA were avoided because additional salts may interfere with the test.
Technique
1. The stock solution of sodium chloride was diluted 1:10 with distilled water to obtain a 1% solution.
2. 12 tubes were used to prepare dilutions as follows:
3. 0.05 ml of human whole blood was added to each of the tubes and was mix immediately by gently inverting several times.
4. The blood from an apparently healthy individual was run in parallel as a control.
5. They were kept at room temperature (18-20°C) for 30 minutes and were remixed and centrifuged.
6. The amount of lysis in each tube was determined calorimetrically using a green filter, at 540 nm. The first tube in the series serves as a blank (0% lysis) as it contains 0.9% saline serves. The 12th tube containing 0.1% saline serves as 100% lysis since this gives complete lysis.
7. This was done in the presence and absence of graded concentrations of the extract.
Calculation
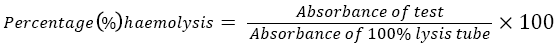
Results
The extract of dark aromatic gotten measured 9.60 g and represented a yield of 19.20%.
Effect of TTE on osmotic fragility (membrane stabilization)–induced haemolysis
The TTE significantly (p<0.05) inhibited hypo tonicity induced haemolysis of erythrocytes in a concentration dependent manner. TTE inhibited haemolysis in a similar manner as Indomethacin. Take for instance, 0.1, 0.2, 0.4 and 0.6 mg/ml of TTE inhibited haemolysis as; 17.3, 38.46, 52.0, and 77.0% respectively. While 0.1, 0.2, 0.4 and 0.6 mg/ml of Indomethacin inhibited hypo tonicity induced haemolysis as; 25.0, 57.7, 67.31 and 73.07% respectively (Table 1).
| Test tube |
Conc. (mg/kg) |
Absorbance |
Inhibition of haemolysis |
| Control |
0.00 |
0.52 0.02 |
0.00 |
| TTE |
0.1 |
0.43 0.00* |
17.30 |
| TTE |
0.2 |
0.32 0.01* |
38.46 |
| TTE |
0.4 |
0.25 0.01* |
52.00 |
| TTE |
0.6 |
0.12 0.00* |
77.00 |
| Indomethacin |
0.1 |
0.39 0.01 |
25.00 |
| Indomethacin |
0.2 |
0.22 0.02 |
57.70 |
| Indomethacin |
0.4 |
0.17 0.01 |
67.31 |
| Indomethacin |
0.6 |
0.14 0.00 |
73.07 |
| Distilled Water |
-- |
0.72 0.22 |
-- |
Table 1: Effect of TTE on osmotic fragility (membrane stabilization)-induced haemolysis.
Effect of TTE on platelet aggregation
Figure 1 shows that the different levels of the TTE hindered platelet aggregation caused by CaCl2 (2 M). The maximum platelet aggregatory function was reached at the 8th min. The inhibition of platelet by the extract corresponds with that of indomethacin. For example, 0.1 mg/ml of the extract gave a percentage inhibition of 78.38, 80.0, 84.37 and 86.67% at different time range (2, 4, 6 and 8 min). 0.2, 0.4, 0.6 and 0.8 mg/ml equally showed the similar inhibition patterns. As the levels of the extract increases, platelet aggregation reduced. For instance, at 8 min, 0.1, 0.2, 0.4, 0.6 and 0.8 mg/ml of the TTE inhibited platelet aggregation as follows; 86.67, 80.3, 67.6, 54.1 and 51.8%, respectively. Meanwhile 0.2 and 0.4 mg/ml of Indomethacin inhibited platelet aggregation as 55.6 and 41.7% respectively at 8 min.
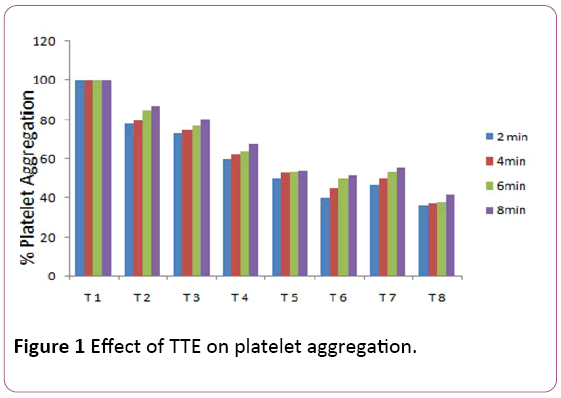
Figure 1: Effect of TTE on platelet aggregation.
• Test tube 1=Normal saline+Calcium Chloride.
• Test tube 2=Normal saline+Calcium Chloride + Plasma (PRP)+0.1 mg/ml.
• Test tube 3=Normal saline+Calcium Chloride + Plasma (PRP)+0.2 mg/ml TTE.
• Test tube 4=Normal saline+Calcium Chloride + Plasma (PRP)+0.4 mg/ml TTE.
• Test tube 5=Normal saline+Calcium Chloride + Plasma (PRP)+0.6 mg/ml TTE.
• Test tube 6=Normal saline+Calcium Chloride + Plasma (PRP)+0.8 mg/ml TTE.
• Test tube 7=Normal saline+Calcium Chloride + Plasma (PRP)+0.2 mg/ml Indomethacin.
• Test tube 8=Normal saline+Calcium Chloride + Plasma (PRP)+0.4 mg/ml Indomethacin.
Effect of extract on phospholipase A2 activity
TTE significantly (p<0.05) inhibited phospholipase A2 activity in a concentration related manner comparable to that of Prednisolone (Figure 2). 1.25 mg/ml of TTE inhibited 99.31% phospholipase A2 activity comparable with 1.0 mg/ml of Prednisolone which inhibited 89.66% phospholipase A2 activity.
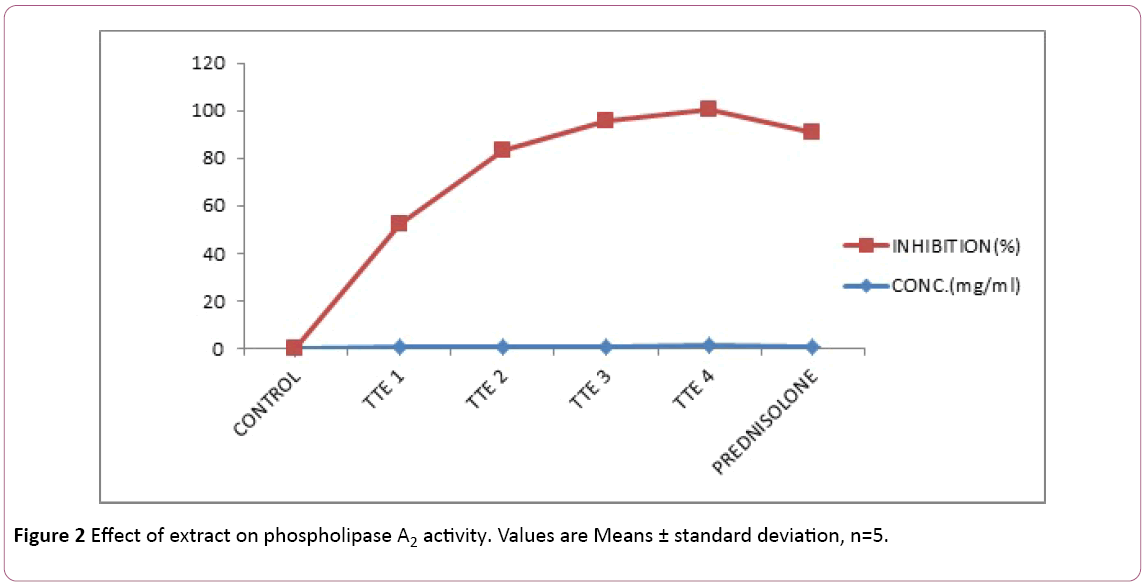
Figure 2: Effect of extract on phospholipase A2 activity. Values are Means ± standard deviation, n=5.
Discussion
The place of platelets in the inflammatory processes is highly recognized, in addition to their function in haemostasis and thrombosis [9]. Platelet accumulates in inflammatory sites together with leukocytes [10] and regulates a variety of inflammatory responses by secreting or activating adhesion proteins, growth factors, chemokines, cytokine-like factors and coagulating factors. These proteins induce widely differing biological activities, including cell adhesion chemotasis, cell survival and proliferation, all of which increase inflammatory process [11].
Figure 1 indicates that the extract significantly (p<0.05) decreases CaCl2-induced platelet aggregation in-vitro. The platelet aggregation decreases with increase in TTE concentration. This indicates that the inhibition of platelet aggregation by TTE is dose dependent implying that as the extract increases, its ability to inhibit platelet aggregation increases too. Since platelet release inducing agents-thrombin, adrenaline, thromboxane A2 (TXA2) and ADP activates the platelet-PG pathway [12] the mechanism for the antiaggregatory activity might be inhibition of the synthesis of thromboxane from arachidonic acid. The inhibitions of platelet aggregation suggest a probable role of TTE in thrombosis, which in turn prevent aggregation of platelet and thromboembolic disorders that can lead to death.
Results on membrane stabilization showed that the extract inhibited haemolysis observed in low osmotic medium. This study is in agreement to Celine et al. and Agar et al. [13,14] that an anti-inflammatory drug stabilizes erythrocyte membranes. This implies that TTE possess properties which reduce more of methaemoglobin than deoxyhaemoglobin. The stabilization of red cell membrane may be linked to high content of phenols in the methanolic extract. This also suggests that the TTE could be used in the management of Spherocytosis and thalassaemia, iron deficiency anaemia. This reason may explain why many pregnant women are fond of it. (Use inform of routine drugs).
Conclusion
TTE significantly reduced phospholipase A2 activity in a concentration related manner related to that of Prednisolone. TTE possess properties which reduce more of methaemoglobin than deoxyhaemoglobin. The stabilization of erythrocyte membrane may be as result of the high content of phenols in the methanolic extract. This also suggests that the TTE could be used in the management of Spherocytosis and thalassaemia, iron deficiency anaemia. This plant can help to stabilize red blood cell membrane integrity anaemia.
Conflict of Interest
The authors declare no conflict of interests.
23266
References
- Okwu DE (2003) The potential of Ocimum gratissimum, Pergularia extensa and Tetrapleura tetraptera as spices and flavouring agents. Nigerian Agriculture Journal 35: 143-148.
- Aladesanmi JA (2007) Tetrapleura tetraptera: Molluscidal activity and chemical constituents. African Journal of Traditional, Complementary and Alternative Medicine 4 (1): 23-36.
- Onwa C Mutua A, Kindi R, Jamnadass RA, Simon A (2009) Agroforest tree database: A tree reference and selection guide version 4.0.
- Akah PA, Alemji JA, Salawu OA, Okoye TC, Offiah NV (2009) Effects of Vernonia amygdalina on biochemical and haematological parameters in diabetic rats. Asian Journal of Medical Sciences 1(3): 108-113.
- Nwodo OFC (1981) Ph.D. Thesis, Elucidation of the nature of pharmacologically active principle extractible from the seeds of Abrus pecatorius. Chelsea College, University of London.
- Born GVR, Cross J (1963) The aggregation of blood platelets. Journal of Physiology 168: 224-226.
- BZ, Ward RFA, Comfurius P, Woodward CB, Van Doenan, et al. (1971) Inflammation, in: Chandra T, Sadique J, Somasundraram S-Effect of Eclipta alba on inflammation and liver injury. Fitoterapia 3: 23-32.
- Ochei J, Kolhatkar A (2007) Medical laboratory science theory and practice. Tata McGraw-Hill, New Delhi pp: 279-294.
- Risa T, Norito K, Eiichiro U, Hideya Y, Masakazu K, et al. (2007) The role of platelet in leukocyte recruitment in chronic contact hypersensitivity induced by repeated elicitation. Am J Pathol 170(6): 219-229.
- Schmitt-Sody M, Klose A, Gottschalk O, Metz P, Gebhard H, et al. (2005) Platelet-endothelial cell interactions in murine antigen-induce arthritis. Rheumatology 44: 885-889.
- Gawaz M, Langer H, May AE (2005) Platelet in inflammation and atherogenesis. J Clin Invest 115: 3378-3384.
- Hatmi M, Haye B, Gavaret JM, Vargaftig BB, Jacquemin C (1991) Alkaline phosphatase prevents platelet stimulation by thromboxane-mimetus. Br J Pharmacol 104(2): 554-558.
- Celine V, Tabassome S, Richard BK (2009) Personalized medicine and anti-platelet therapy: Ready for prime time. Eur Heart J 30:(16): 1943-1963.
- Agar NS, Ogawa SS, Cauaghan O, Hume ID (1998) The effect of eucalyptus oil on the erythrocytes of koalas. International Journal of Comparative Haematology 8: 225-229.