Key words
|
| |
| Pemetrexed disodium; HPLC; UV Spectrophotometry; Validation; Student’s t-test; Pharmaceutical formulation |
| |
|
INTRODUCTION
|
| |
| Pemetrexed disodium has the chemical name N-[4- [2-(2-amino-4,7-dihydro-4-oxo-1H-pyrrolo [2,3-d] pyrimidin-5-yl)-ethyl]-benzoyl]-L-glutamic acid disodium salt, heptahydrate. Pemetrexed disodium is a folate analog metabolic inhibitor that exerts its action by disrupting folate-dependent metabolic processes essential for cell replication.1 |
| |
| In-vitro studies have shown that Pemetrexed inhibits thymidylate synthase (TS), dihydrofolate reductase (DHFR), and glycinamide ribonucleotide formyltransferase (GARFT), which are folatedependent enzymes involved in the de-novo biosynthesis of thymidine and purine nucleotides.2,3 Cells that are resistant to antifolates are generally less resistant to Pemetrexed, irrespective of the mechanism of resistance. It is used in treatment of locally advanced or metastatic nonsquamous nonsmall cell lung cancer (NSCLC), breast cancer, malignant Pleural mesothelioma, colorectal cancer and pancreatic cancer.4,5 |
| |
| Analytical techniques such as gradient HPLC, LC-MS are available for the estimation of Pemetrexed disodium in human plasma and urine.6,7 So far to our present knowledge no HPLC and UV spectrophotometric methods for estimation of Pemetrexed disodium in pharmaceutical formulation are available in the literature. It is felt necessary to develop HPLC and UV methods for the quantitative determination of Pemetrexed disodium. The current research work deals with development and validation of HPLC and UV methods. Method validation is done as according to International Conference on Harmonization guideline.8 |
| |
|
RP-HPLC METHOD
|
| |
|
Experimental
|
| |
|
Chemicals and reagents
|
| |
| Pemetrexed disodium was supplied by Intas pharmaceutical limited, Ahmedabad, as gift sample. Pemetrexed disodium powder for infusion (Pemmet infusion) was purchased from market. Acetonitrile, ortho-phosphoric acid, disodium hydrogen phosphate (HPLC grade, Finar Chemicals Ltd, Ahmedabad, India) and water (HPLC grade, RFCL limited, New Delhi, India) were used for study. |
| |
|
Instrumentation
|
| |
| The LC system used for method development and method validation was a Shimadzu HPLC instrument (LC_2010 CHT) equipped with prominence diode array detector (SPD-M20A). The output signal was monitored and processed using LC Solution software. An analytical balance (Acculab ALC-210.4, Huntingdon Valley, PA), pH meter (Thermo electron crop, Pune, India) and sonicator (EN 30 US, Enertech fast clean, Mumbai, India) were used for study. |
| |
|
Preparation of standard solution (1000μg/ml)
|
| |
| Accurately weighed quantity of 100 mg Pemetrexed disodium standard was transferred into 100 ml volumetric flask and dissolved and diluted up to the mark with HPLC grade water to give a stock solution having strength 1000μg/ml. |
| |
|
Preparation of sample stock solution
|
| |
| Powder for infusion equivalent to 50 mg of Pemetrexed disodium was weighed and transferred into a 50 ml of volumetric flask, dissolved and diluted up to the mark with HPLC grade water. This solution was filtered using whatman filter paper no.42. (1000 μg/ml) |
| |
|
Method validation
|
| |
|
Linearity and range
|
| |
| The linearity was determined by analyzing 6 independent levels of calibration curve in the range of 20-120 ?g/ml. 20 ?l of all these solutions were injected separately into HPLC column and peak area of each solution was measured at 254 nm. The calibration curve of peak area vs. concentration was plotted and correlation co-efficient and regression line equation for Pemetrexed disodium were determined. |
| |
|
Precision
|
| |
| Intra-day precision was determined by analyzing Pemetrexed disodium (20-120 ?g/ml) at three different time points on the same day and inter-day precision was determined by analyzing Pemetrexed disodium (20-120 ?g/ml) at three different time points on different days and %RSD was calculated. |
| |
|
Accuracy
|
| |
| Accuracy was determined by performing recovery studies by spiking different concentrations of pure drug in the pre-analyzed powder for infusion samples within the analytical concentration range of the proposed method at three different set at level of 50%, 100% and 150%. The amount of Pemetrexed disodium was calculated at each level and % recoveries were computed. |
| |
|
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
|
| |
| The LOD and LOQ were estimated from the set of 5 calibration curves used to determine method linearity. |
| |
| LOD= 3.3*σ/S and LOQ= 10*σ/S |
| |
| Where, σ = the standard deviation of y-intercepts of regression lines |
| |
| S = the slope of the calibration curve |
| |
|
Robustness
|
| |
| The robustness was checked by small but deliberate change in three parameters like mobile phase flow rate (±0.1ml/min), mobile phase composition (±2.0 ml organic modifier) and pH (±0.20 units). |
| |
|
Solution stability
|
| |
| Standard and sample solution stability was evaluated at room temperature for 24 hours. |
| |
|
Analysis of marketed formulation (Pemmetpowder for infusion) by proposed method
|
| |
| From the sample stock solution, 1 ml was taken in 10 ml volumetric flask and diluted up to the mark with HPLC grade water. (100 μg/ml) |
| |
Result and discussion
|
| |
|
Linearity and range
|
| |
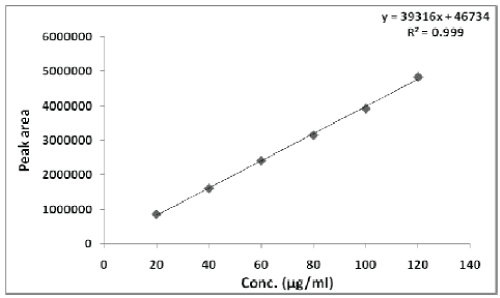
| The linearity of Pemetrexed disodium was found to be in the range of 20-120 μg/ml with correlation coefficient 0.999. Calibration data with %RSD is shown in Table 2 and calibration curve is shown in Figure 3. |
| |
|
Precision
|
| |
| In case of intra-day precision, %RSD was found to be in the range of 0.21-0.68. |
| |
| In case of inter-day precision, %RSD was found to be in the range of 0.44-0.88. |
| |
|
Accuracy
|
| |
| % Recoveries for Pemetrexed disodium were found to be 98.80-101.87. |
| |
|
Limit of Detection and Limit of Quantitation
|
| |
| LOD and LOQ were found to be 0.445 μg/ml and 1.348 μg/ml, respectively. |
| |
|
Robustness
|
| |
| Overall %RSD was found to be 0.40. The robustness data are shown in table for three parameters like mobile phase flow rate, mobile phase composition and pH. Robustness data is shown in Table 3. |
| |
|
Solution stability
|
| |
| The %RSD of the assay of Pemetrexed disodium during solution stability experiment was within 1.0%. No significant change was observed in assay content of Pemetrexed disodium during solution stability experiment. The solution stability experiment data confirmed that standard and sample solutions used during assay were stable up to 24 hr. |
| |
|
Analysis of marketed formulation (Pemmetpowder for infusion) by proposed method
|
| |
| The percentage of Pemetrexed disodium in marketed formulation (Pemmet-powder for infusion) was calculated from the calibration curve of Pemetrexed Disodium. %Assay was found to be 99.27% as shown in Table 4. |
| |
UV SPECTROPHOTOMETRIC METHOD
|
| |
|
Experimental
|
| |
|
hemicals and reagents
|
| |
| Distilled water was used throughout UV spectrophotometric method development and validation. |
| |
|
Instrumentation
|
| |
| UV spectrophotometric method was performed on double beam UV-visible spectrophotometer (Shimadzu, model 1700) having two matched quartz cells with 1 cm light path. |
| |
|
Selection of solvent
|
| |
| Distilled water was selected as ideal solvent for spectrophotometric analysis of Pemetrexed disodium |
| |
|
Preparation of standard stock solution (1000μg/ml)
|
| |
| Accurately weighed quantity of 100 mg Pemetrexed disodium reference standard was transferred into 100 ml volumetric flask and dissolved and diluted up to the mark with distilled water to give a stock solution having strength 1000μg/ml. 100 ?g/ml working standard solution was prepared by diluting 1 ml of stock solution to 10 ml with distilled water. |
| |
|
Preparation of sample stock solution (100μg/ml)
|
| |
| Powder for infusion equivalent to 10 mg of Pemetrexed disodium was weighed and transferred into a 100 ml of volumetric flask, dissolved and diluted up to the mark with distilled water. This solution was filtered using whatman filter paper no.42. (100 μg/ml) |
| |
|
Validation of UV spectrophotometric method Linearity and range
|
| |
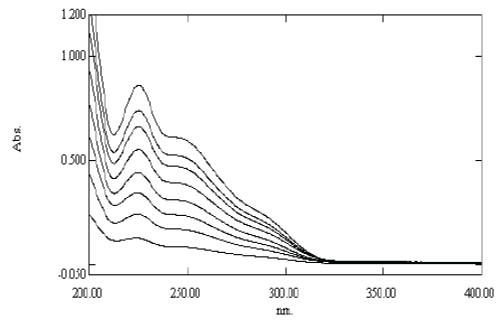
| The linearity was determined by analyzing 8 independent levels of calibration curve in the range of 2-16 μg/ml. Absorbance of each solution against distilled water was recorded at 225 nm. The calibration curve of absorbance vs. concentration was plotted and correlation co-efficient and regression line equation for Pemetrexed disodium were determined. |
| |
|
Precision
|
| |
| Intra-day precision was determined by analyzing Pemetrexed disodium (2-16 μg/ml) at three different time points of the same day and inter-day precision was determined by analyzing Pemetrexed disodium (2-16 μg/ml) at three different time points on different days and %RSD was calculated. |
| |
|
Accuracy
|
| |
| Accuracy was determined by performing recovery studies by spiking different concentrations of pure drug in the pre-analyzed powder for infusion samples within the analytical concentration range of the proposed method at three different set at level of 50%, 100% and 150%. The amount of Pemetrexed disodium was calculated at each level and % recoveries were computed. |
| |
|
Limit of Detection (LOD) and Limit of Quantitation (LOQ)
|
| |
| The LOD and LOQ were estimated from the set of 5 calibration curves used to determine method linearity. |
| |
| LOD= 3.3*σ/S and LOQ= 10*σ/S |
| |
| Where, σ = the standard deviation of y-intercepts of regression lines |
| |
| S = the slope of the calibration curve |
| |
|
Analysis of marketed formulation (Pemmetpowder for infusion) by UV spectrophotometric method
|
| |
| From the sample stock solution, 1 ml was taken in 10 ml volumetric flask and diluted up to the mark with distilled water. (10 μg/ml) |
| |
Result and discussion
|
| |
|
Linearity
|
| |
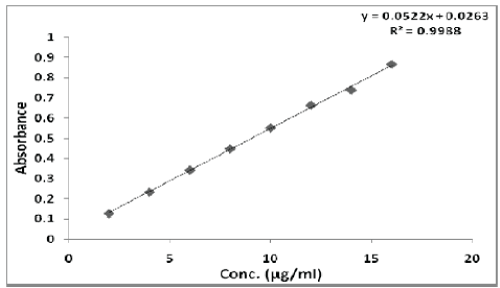
| The linearity of Pemetrexed disodium was found to be in the range of 2-16 μg/ml with correlation coefficient 0.9988. Calibration data with %RSD is shown in Table 6 and calibration curve is shown in Figure 5. |
| |
|
Precision
|
| |
| In case of intra-day precision, %RSD was found to be in the range of 0.21-0.79. |
| |
| In case of inter-day precision, %RSD was found to be in the range of 0.61-1.28. |
| |
|
Accuracy
|
| |
| Accuracy of the method was confirmed by recovery study from marketed formulation at three level of standard addition. % Recovery for Pemetrexed disodium was found to be 99.33-101.28. |
| |
|
Limit of Detection and Limit of Quantitation
|
| |
| LOD and LOQ were found to be 0.209?g/ml and 0.632?g/ml, respectively. |
| |
|
Analysis of marketed formulation (Pemmetpowder for infusion) by UV spectrophotometric method
|
| |
| The percentage of Pemetrexed disodium in marketed formulation (Pemmet-powder for infusion) was calculated from the calibration curve of Pemetrexed disodium. %Assay was found to be 100.44% as shown in Table 7. |
| |
|
Comparison between HPLC method and UV method
|
| |
| The proposed analytical methods were compared using statistical analysis. The Student’s t - test was applied and does not reveal significant difference between the experimental values obtained in the sample analysis by the two methods. The calculated t -value (tcalc= 0.002) was found to be less than the critical t -value (tcrit= 1.943) at 5% significance level. |
| |
|
CONCLUSION
|
| |
| A specific, accurate, precise and robust isocratic HPLC method has been developed for the determination of Pemetrexed disodium in active pharmaceutical ingredient and powder for infusion. A simple, rapid, economic, accurate and precise UV spectrophotometric method has been developed for the determination of Pemetrexed disodium in active pharmaceutical ingredient and powder for infusion. The proposed methods can be used for the drug analysis in routine quality control. |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
 |
 |
 |
 |
| Table 5 |
Table 6 |
Table 7 |
Table 8 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |











