Introduction
When skin becomes broken due to an injury or trauma to the tissue, the normal wound healing process begins. Basically, three phases of wound healing occur in healthy individuals, which include the inflammatory, proliferation and remodeling phase. Chronic wounds seem to be arrested in a stage dominated by inflammatory processes [1]. Bacteria can establish a complex community within the wound. These communities are organized as biofilms. Wound bacterial biofilms offer an explanation for the pathophysiology of non-healing wounds. Aerobic bacteria such as Pseudomonas aeruginosa (P. aeruginosa) and Staphylococcus aureus (S. aureus) have long been recognized as major wound pathogens. The ability of colonizing bacteria to establish and proliferate in a biofilm in the wound leads to the increasing emergence of multidrug-resistant P. aeruginosa and S. aureus. This might contribution to the lack of successful antibiotic treatment [2,3].
The bacteria in a micro-colony divide and reach a critical density, which has been termed a quorum. These cells produce extracellular molecules used to communicate from cell to cell (quorum sensing molecules), that is important for biofilm development. Therefore, manipulating the cell-cell signaling of the bacterial community can also alter the defenses and virulence of the bacterial community [4,5]. Controlling bacteria in wounds is a vital factor in achieving wound healing. Stringent management regimens that combine multiple strategies into a single, concomitant approach, aimed at promoting the patient’s normal healing functions and also killing the bacteria and degrading the community, which has afforded added defenses to the bacteria are required for chronic wound healing [5]. Combining anti-biofilm strategies with traditional strategies as debridement in non-healing and potentially infected wounds can be used to weaken the biofilm until it reaches a level at which the impaired host’s immunity can fight off the chronic infection. The most exciting area of research in antibiofilm agents is quorum sensing inhibitors [6].
Pantothenic acid (B5 vitamin) is essential to normal epithelial function. It is a component of coenzyme A, which serves as a cofactor for a variety of enzyme-catalyzed reactions that are important in the metabolic processes. The topical use of dexpanthenol, the stable alcoholic analog of pantothenic acid, is based on good skin penetration and high local concentrations. Topical dexpanthenol acts like a moisturizer, improving stratum corneum hydration, maintaining skin softness and elasticity. The stimulation of epithelization, granulation and mitigation of itching were the most prominent effects of formulations containing dexpanthenol [7].
Propolis is a multifunctional substance used by bees to maintain the safety of their hives. It is worldwide used for its potential therapeutic effects. Propolis is a chemically very complex resinous bee product collected by bees from tree buds. The main constituents of propolis are beeswax, resin and volatiles. The insects secrete beeswax, while the other two constituents are obtained from plants [8]. Along with other honey bee products, propolis has outstanding therapeutic properties. It has been shown to exhibit pharmacological activities such as antibacterial, antiviral, antifungal, antioxidant, anti-inflammatory, anti-cariogenic and immune-stimulating properties [9-13].
As the in-depth knowledge of chronic wounds and searching new ways of fighting biofilm is urgently required and would significantly improve treatment and prognosis. The aim of the present study involved practical management of Pseudomonas and Staphylococcus wound infections through determination of activity of different remedies such as dexpanthenol and propolis on the selected pathogens and study of the effects of drug combinations on the formation of single and mixed bacterial biofilms as well as their effects on induced infected wounds in rats (in vivo models).
Methods
Following up on our previous study [14], Six Staphylococcus and Pseudomonas clinical strains selected from 80 strains isolated from wound swab cultures at the Microbiology Laboratory of Medical Research Institute, Alexandria University through 2007-2008 were included in this study. Two were present as single entities and four were in mixture of two each. Isolates were identified using conventional methodologies. These strains were selected according to their biofilm forming capacity (strong biofilm forming single isolates) and the magnitude of change in their biofilm production (for the isolate mixtures) as tested by modified microtiter plate method [15]. Possible mechanisms involved in such change among which N- acylhomoserine lactone (quorum sensing molecule) production intensity in Pseudomonas isolates were previously investigated [14]. The tested isolates were: N31 P. aeruginosa [strong biofilm former, optical density (O.D.)= 0.39; N- acyl- homoserine lactone producer, O.D.= 0.353], N31 mixture isolates (O.D.= 0.869) [N31 P. aeruginosa and N31 S. aureus (weak biofilm former, O.D.=0.093)], N6 S. aureus [strong biofilm former, O.D.=0.508]; N6 mixture isolates (O.D.= 0.216) [N6 S. aureus and N6 P. aeruginosa (weak biofilm former O.D.= 0.074; N- acyl-homoserine lactone producer, O.D.= 0.467)], N4 P. aeruginosa [strong biofilm former O.D.= 0.4; N- acyl-homoserine lactone producer O.D.= 0.467], N25 S. aureus [strong biofilm former, O.D.= 0.414]. Antimicrobial susceptibility testing of the isolates was performed by Kirby-Bauer method onto Mueller-Hinton agar and interpreted according to Comité de l’Antibiogramme de la Société Française de Microbiologie (CASFM) 2008. In the present study, these selected strains were challenged against certain antibiotics applied alone and in combination with local wound healing agent as dexpanthenol and propolis extract.
Effect of dexpanthenol on the minimum inhibitory concentration [MIC] of four selected topical antimicrobial agents
Gentamicin, ciprofloxacin, oxytetracycline (El-pharaonia pharmaceutical company, Alexandria, Egypt) and colistin sulphate (Arab company for gelatin and pharmaceutical products, Alexandria, Egypt) as powder were used to prepare stock solutions. The rationale behind using these topically applied antibiotics was as follows: Gentamicin and oxytetracycline are widely used as topical antimicrobials in Egypt, for colistin it is coming back into action [16], and for ciprofloxacin; it is a recently topically applied antimicrobial in treatment of soft tissue infections [17]. Dexpanthenol gel (Luna cosmetics company, Egypt) was used to prepare stock solutions.
Preparation of Stock Solutions
Stock solutions of the selected antibiotics were prepared as follows
Four hundred milligrams of gentamicin sulphate powder were dissolved in sterile distilled water to prepare 0.4% solution and the same was done for colistin sulphate and oxytetracycline. For ciprofloxacin, 400 mg of the drug was dissolved in minimal amount of 0.1N NaOH, and then completed to the required volume with sterile distilled water. Stock solution of dexpanthenol was prepared as follows: Twenty grams of dexpanthenol were dissolved in sterile distilled water to prepare 20% dexpanthenol solution.
Checkerboard method
MICs of each of the selected antibiotics and dexpanthenol alone and in combination with the antibiotics were determined by broth micro-dilution technique using checkerboard method [15] carried out in 96 wells sterile microtiter plates (Greiner bio 1, cellstar). Antibiotics were diluted to a final concentration ranging between 1000 and 0.9 μg/ml and distributed along the horizontal wells (2-12), 50 μl/well. Vertical wells received final concentrations of dexpanthenol [3%, 2. 5%, 2%, 1.5%, 1% and 0.5%] (50μl/well). A1 served as negative control (100μl sterile distilled water and100μl un-inoculated double strength broth). Each of the 84 wells received 100μl of sterile double strength broth inoculated with the organism (s) under test (106 CFU/ ml), OD=0.2 at 600 nm. H1 served as positive control (100μl sterile distilled water and100μl inoculated double strength broth). Plates were covered and incubated at 35-37°C for 24 hours and MIC was calculated as the lowest antimicrobial agent concentration with lack of visual turbidity. Results of the combinatorial effect of dexpanthenol and the antimicrobial agent were interpreted as activity index as follows: Activity index = MIC of the antimicrobial agent in the combination/ MIC of the antimicrobial agent alone. The obtained activity index values were translated into log activity index values for ease of interpretation. The experiment was repeated three times and results were reproductive.
Determination of antibiofilm activity of certain antibiotics alone and in combination with Dexpanthenol against selected bacterial isolates using modified microtiter plate method [15,18]
The same Checkerboard arrangement was used as mentioned previously. After 24 hours incubation, plates were emptied and washed three times with sterile saline and fixed with 200 μl of 99% methanol for 15 min. Wells were emptied and air dried. After that, 200 μl of 2% crystal violet solution was dispersed in each well, left for 5 minutes then washed and dried. Each well was eluted with 160 μl of 33% glacial acetic acid and read using ELISA plate reader at 590 nm. Results were interpreted and plotted into Figures.
Determination of efflux inhibition capacity of Dexpanthenol
A simple qualitative method according to Martins et al. (2009) [19] was applied. It was done by a 96-well micro-plate screening assay, where serial dilutions of ethidium bromide (EB) starting at 4μg/ml up to 0.003 μg /ml, were dispensed to the wells (2-12) in the rows A, B, C. The wells B1- B12 and C1 -C12 received dexpanthenol with a final concentration of 0.5 % and 3 %, respectively. Then an inoculum of 106 CFU /ml of the selected organisms in double strength broth was added to the wells A (2-12), B (2-12) and C (2 -12). A1 (EB, sterile distilled water and inoculated broth), B1 and C1 (EB, dexapanthenol and un-inoculated broth) served as controls. The plates were incubated at 35-37°C, overnight and the lowest concentration of EB that promotes fluorescence under ultraviolet trans-illuminator was visually interpreted and photographed.
Determination of antimicrobial activity of propolis extract
The chemical composition of propolis (American propolis) is four flavonoids: kaempferol, galangin, 3,3’-dimethoxyquercetin and 3-methoxykaempferol, also contains caffeic acid, chrysin, ferulic acid [20] (Arab company for gelatin and pharmaceutical products, Alexandria, Egypt)
Preparation of propolis extract
Twenty grams of propolis powder was mixed with 100 ml of 95% ethanol to obtain 20% W/V propolis suspension. Extraction was carried at room temperature in dark for 7 days with frequent shaking. After extraction the mixture was filtered and the filtrate was designated as ethanolic extract of propolis [EEP] [21]. Antimicrobial activity of EEP was determined by agar diffusion against N31Staphylococcus and Pseudomonas isolates as representative of Gram positive and Gram negative bacteria, respectively [21] using EEP in different dilutions (10, 5, 2.5, 1.25 and 0.625%).
Determination of the MIC of EEP by agar dilution technique
The test plates were prepared with 19 ml of Mueller-Hinton agar and 1 ml of 2-fold serial dilution of EEP in sterile distilled water starting at 10 mg/ml and up to 0.019 mg/ml. The plates were spot inoculated with 10 μl of 106cells/ml of the tested isolates and incubated for 24 hours at 35-37°C. The MIC was defined as the lowest concentration that prevented visible growth of micro-organisms spots [21]. Parallel control plates using ethanolic solutions in the same concentrations (95% ethanol in serial dilutions) were done.
Determination of the effect of EEP on different antimicrobial agents
Kirby-Bauer method with modifications according to Stepanovic et al. (2003) [21] instead of broth micro-dilution technique using checkerboard method due to clouding of the wells upon EEP addition. The test was carried out using the six bacterial isolates. The following antibiotics were challenged: polymyxin B, colistin sulphate, vancomycin, Sulphamethoxazole- trimethoprim, erythromycin, tetracycline, chloramphenicol, bacitracin, fusidic acid, ampicillin-sulbactam, amoxicillinclavulanic acid, methicillin, cefaclor, cefoperazone, cefipime, imipenem, ciprofloxacin, ofloxacin, levofloxacin, gentamicin, neomycin, tobramycin (Oxoid Ltd., UK). Two milliliters of appropriate concentration [sub-inhibitory concentration (0.25 MIC of EEP of the corresponding isolate)] of EEP were mixed with 38 ml of Mueller-Hinton agar to obtain concentration of EEP that did not inhibit microbial growth. Parallel controls with same concentrations of the ethanolic solution were carried out. The plates were incubated and diameter of inhibition zones was measured and compared to that in absence of EEP and plotted into Figures.
Determination of antibiofilm activity of EEP
Biofilm assay was performed according to the modified microtiter plate methods [15] using a range of final EEP concentrations from 0.05 to 100 mg/ml. Parallel controls of the corresponding ethanolic solution in two fold serial dilutions was carried out.
In vivo study of drug combination effects on the formation of mixed bacterial biofilms
Induction of wound infection in rat using a mixture of P. aeruginosa and S. aureus (N31) culture-mate isolates
To establish an animal model of P. aeruginosa and S. aureus biofilm associated with wound infection and investigate the pathogenic effects of biofilm and the outcome of different treatment regimen, Four Para-vertebral incision wounds; were made by sterile surgical blades on the dorsal surface of 30 Sprague- Dawley albino female rats of 3 months age and weighing about 170 grams. All surgical procedures were carried out under anesthesia using thiopental 40 mg/kg body weight administered intraperitoneally. Each wound received 50 μl of mixture of 106 cells/ml P. aeruginosa and 106 cells/ml S. aureus; originally isolated together from a wound. The wounds were stitched under aseptic conditions [22]. All experimental procedures were approved by the Institutional Ethics Committee in accordance with the Gide to the Care and Use of Experimental Animals by Canadian Council for Animal Care (1993).
Treatment of wounds using two antibiotics each alone or in combination with dexpanthenol: Preparation of gel
Gel was prepared using carbopol 934 NF (Luna cosmetics company, Egypt) in a 0.5% concentration. Gel was soaked overnight in the prepared solution of antibiotic and alkalinized in the next day with triethanolamine or 0.1 N NaOH except for ciprofloxacin gel which was prepared in a 0.1 N NaOH solution from the start. The dose of dexapanthenol was dissolve in the gel, mixed and kept in small containers at room temperature. The prepared EEP gel darkened upon standing as a result we continued in the in vivo experiment with dexapanthenol.
Rat experiment: Rats were divided into six groups; five rats each; that received the following treatments: Group 1: Carbopol gel 0.5% base, Group 2: 4% dexpanthenol gel (Dexapanthenol in the chosen concentration here has no cytotoxic activity according to Klöcker et al. (2004) [23] who reported that Dexpanthenol is considered to be safe up to 5%.), Group 3: 0.4% gentamicin gel, Group 4: 0.4 % gentamicin and 4% dexpanthenol gel, Group 5: 0.4% ciprofloxacin gel, Group 6: 0.4% ciprofloxacin and 4% dexpanthenol gel. One rat was kept as no treatment control and sacrificed under anesthesia on the second day post infection. All treatments began from day one post infection. Gels were applied once daily for all rats until complete healing of the wounds, then rats were sacrificed under anesthesia and the tested skin areas were excised and kept in freshly prepared 10% formaldehyde solution and examined histopathologically [22].
Results
Effect of dexpanthenol on MIC of the selected topically applied antibiotics
Results of the combinatorial effect of dexpanthenol and the antimicrobial agent were interpreted as activity index instead of fractional inhibitory concentration (FIC) as dexpanthenol showed no antimicrobial activity under the tested concentration range. The obtained activity index values were translated into log activity index values for ease of interpretation.
Effect of different dexpanthenol concentrations on the MIC of four topically applied antibiotics against P. aeruginosa present in isolate mixture N31
The effect of dexpanthenol started at concentration 1.5 % showed 2 fold decreases in the MIC of all tested antibiotics and continued till reach 16 fold and 4 fold decreases in case of gentamicin and ciprofloxacin respectively at 3% dexpanthenol. All dexpanthenol concentrations showed 2 fold decreases in MIC for oxytetracycline and colistin (Table 1). On calculation of log activity indices of the 4 selected antibiotics, the synergistic effect of dexpanthenol was evident in case of gentamicin sulphate (Figure 1a).
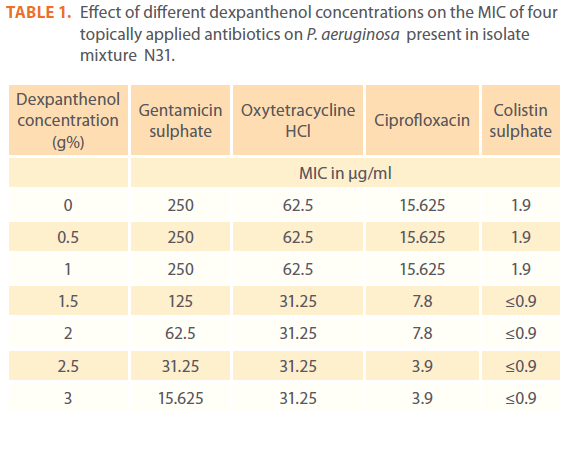
Table 1: Effect of different dexpanthenol concentrations on the MIC of four topically applied antibiotics on P. aeruginosa present in isolate mixture N31.
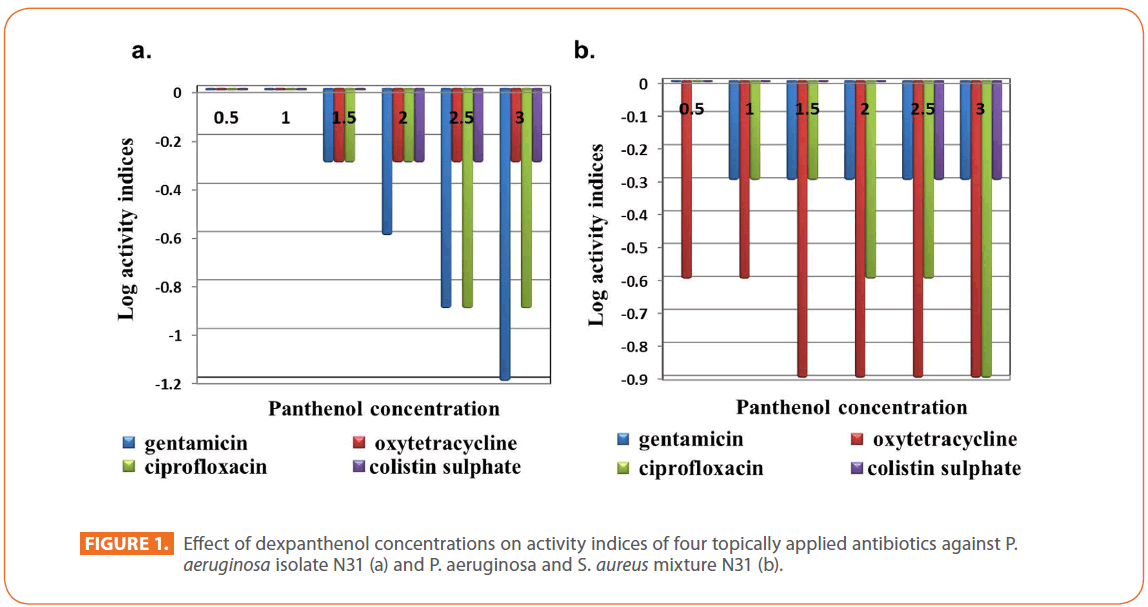
Figure 1: Effect of dexpanthenol concentrations on activity indices of four topically applied antibiotics against P. aeruginosa isolate N31 (a) and P. aeruginosa and S. aureus mixture N31 (b).
Effect of different dexpanthenol concentrations on the MIC of four topically applied antibiotics against P. aeruginosa and S. aureus isolates mixture N31
A decrease of the MIC started at 0.5% for oxytetracycline (MIC=125 μg/ml) and at 1% for gentamicin (MIC=1000 μg/ml) and ciprofloxacin (MIC=62.5μg/ml), reaching 8 fold decrease in MIC at 3% dexpanthenol for oxytetracycline and ciprofloxacin while remained constant at 2 fold for gentamicin. On the other hand, 2 fold decrease in MIC for colistin starts at 2.5%. Synergism was evident for oxytetracycline and ciprofloxacin reaching its maximum effect at 1.5% for oxytetracycline and 3% for ciprofloxacin (Figure 1b).
Effect of different dexpanthenol concentrations on the MIC of three topically applied antibiotics on S. aureus present in isolate mixture N6
An abrupt decrease in MIC of oxytetracycline occurred at 2% dexpanthenol that reached 32 fold, while a gradual decrease occurred in MIC from 1.5% dexpanthenol for gentamicin and from 2% dexpanthenol for ciprofloxacin up to 8 fold for gentamicin and 4 fold for ciprofloxacin at 3% dexpanthenol (Table 2). Synergistic effect of dexpanthenol started at 1.5% with gentamicin and 2% with other tested antibiotics (Figure 2a).
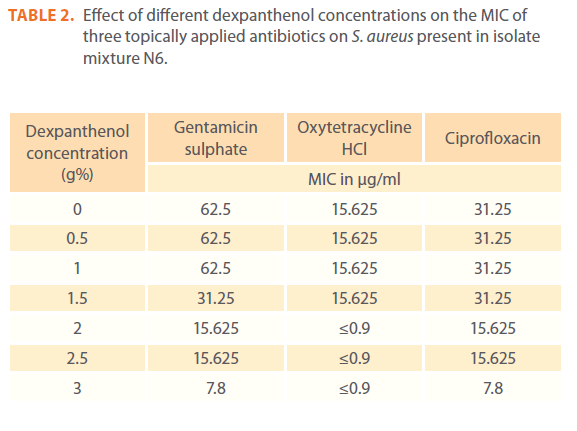
Table 2: Effect of different dexpanthenol concentrations on the MIC of three topically applied antibiotics on S. aureus present in isolate mixture N6.
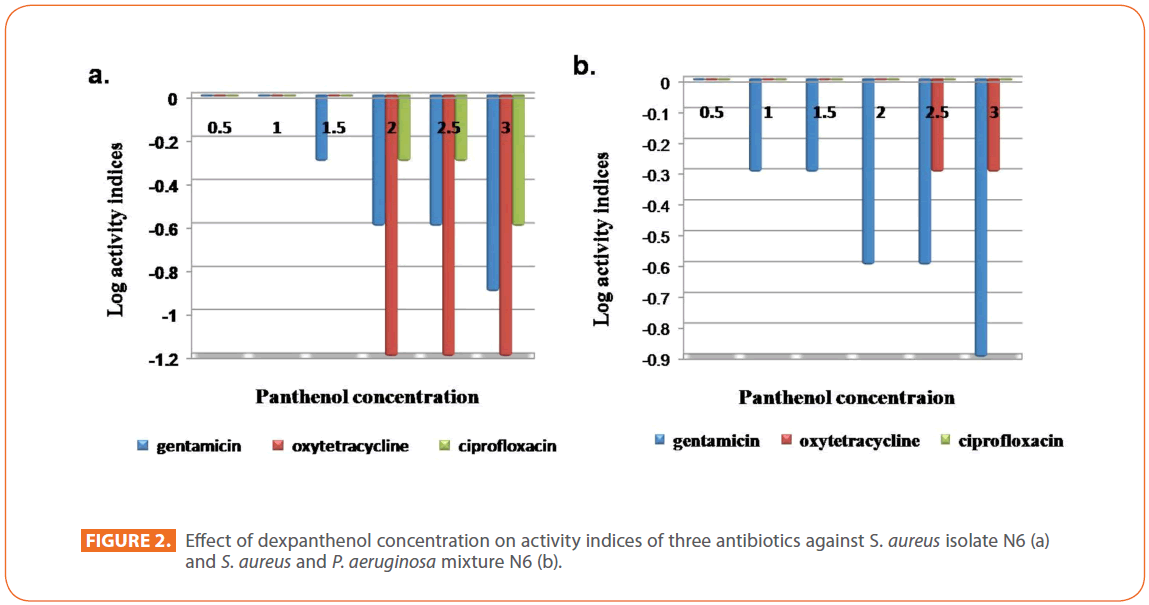
Figure 2: Effect of dexpanthenol concentration on activity indices of three antibiotics against S. aureus isolate N6 (a) and S. aureus and P. aeruginosa mixture N6 (b).
Effect of different dexpanthenol concentrations on the MIC of three topically applied antibiotics against S. aureus and P. aeruginosa isolates mixture N6
A 2 fold decrease in the MIC of gentamicin (MIC=500 μg/ml) and oxytetracycline (MIC=62.5 μg/ml) started at 1% and 2.5% respectively, the decrease remained constant for oxytetracycline and reached 8 fold at 3 % dexpanthenol for gentamicin. On the other hand, it had no apparent effect on the MIC of ciprofloxacin (MIC=31.25μg/ml). Synergistic Effect of dexpanthenol was more apparent for gentamicin reaching its maximum at 3% dexpanthenol (Figure 2b).
Effect of different dexpanthenol concentrations on the MIC of four topically applied antibiotics on P. aeruginosa isolate N4
A 2 fold decrease in the MIC of gentamicin and ciprofloxacin (62.5 up to 31.25 μg/ml and 1.9 up to ≤0.9 μg/ml, respectively) started at 0.5 % dexpanthenol and remained constant for the different concentrations applied for dexpanthenol, while reached 4 fold decrease (15.625 μg/ml) at 3% dexpanthenol in case of gentamicin. Dexpanthenol at different concentrations had no effect on the MIC of colistin and oxytetracycline. An evident synergistic effect of dexpanthenol with gentamicin sulphate appeared at concentrations ranged between 1 and 3% and negligible for the other antibiotics.
Effect of different dexpanthenol concentrations on the MIC of three topically applied antibiotics on S. aureus isolate N25
Two fold decrease started at 0.5% and 1% for ciprofloxacin (31.25 up to 15.625 μg/ml) and gentamicin (250 up to 125 μg/ ml) respectively and reached 8 fold for ciprofloxacin (3.9 μg/ ml) and 4 fold for gentamicin (62.5 μg/ml) at 3 % dexpanthenol , an abrupt decrease (32 fold) was noticed in oxytetracycline (31.25 up to 0.97 μg/ml) upon dexpanthenol addition. Maximum synergistic effect of dexpanthenol with oxytetracycline was apparent in all tested dexpanthenol concentrations. These results were found to be statistically significant by Paired T test and confidence interval (CI) = 95% using MiniTab 16 taking effect of dexpanthenol on activity of applied antibiotics (P=0.03 to < 0.3)
Effect of presence of dexpanthenol alone and in combination with the four topically applied antibiotics on the biofilm forming capacity of the tested isolates
Regarding gentamicin sulphate, in case of Pseudomonas isolate N31, a great decrease in biofilm formation by gentamicin alone occurred gradually with the lowest value at 3.9 μg/ ml, this decrease in biofilm was augmented by the presence of dexpanthenol at the different concentrations used. Concerning the biofilm forming capacity of isolates mixture N31, an abrupt decrease in the biofilm formation was observed among the different concentrations of gentamicin alone and in combination with dexpanthenol. Dexpanthenol alone showed also a great decrease in the biofilm formation in all concentrations under test (Figure 3a). The same was observed for Pseudomonas isolate N4 and Staphylococcus isolate N25. In case of Staphylococcus isolate N6, a marked inhibition of the biofilm formation capacity was observed among the different concentrations used for dexpanthenol and gentamicin. Dexpanthenol alone was very effective at preventing the biofilm formation at all concentrations used (1-3%). On the other hand, slight decrease in the biofilm forming capacity of isolates mixture N6 was observed among the different concentrations of dexpanthenol used either alone or in combination with gentamicin reach maximum decrease at 125μg / ml and above, while gentamicin alone at low concentrations showed an enhancement of the biofilm formed mass that started to decrease by increasing concentration (Figure 3b).
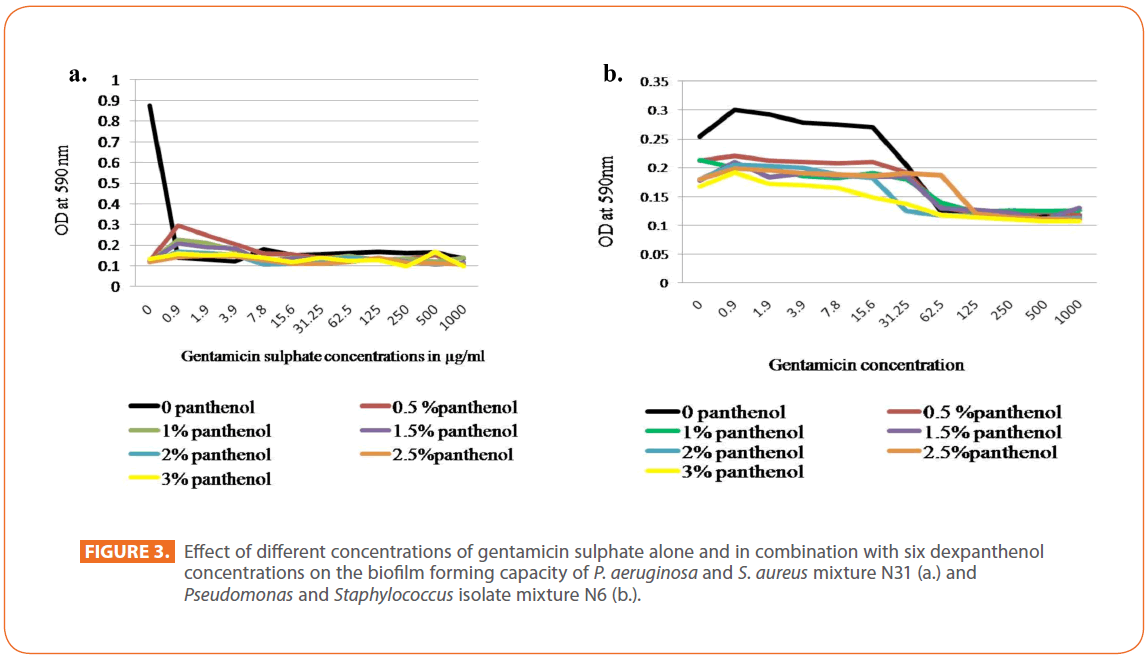
Figure 3: Effect of different concentrations of gentamicin sulphate alone and in combination with six dexpanthenol concentrations on the biofilm forming capacity of P. aeruginosa and S. aureus mixture N31 (a.) and Pseudomonas and Staphylococcus isolate mixture N6 (b.).
Concerning oxytetracycline Hcl, a gradual decrease in biofilm formed mass by the P. aeruginosa N31 was achieved in presence of oxytetracycline; presence of dexpanthenol potentiated the antibiotic effect in prevention of biofilm formation. For isolates mixture N31, dexpanthenol led to a drastic decrease in biofilm forming capacity of the isolate under test at all its concentrations, maximum effect in combination with oxytetracycline is at 15.6 μg/ml and above. A gradual decrease in the biofilm forming capacity of the tested isolate was detected by oxytetracycline alone and weak biofilm was formed under the effect of presence of dexpanthenol alone and when combined with oxytetracycline in case of S. aureus present in mixture N6. The same was observed for the isolates mixture N6. A steep decrease in the formed biofilm mass of Pseudomonas isolate N4 was noticed and started from antibiotic concentration, 1.9 μg/ml, a negligible biomass is allowed to form. Furthermore, a more intensified behavior is noticed at different dexpanthenol concentrations alone and in combination with antibiotic. The same was applied for Staphylococcus isolate N25.
Regarding ciprofloxacin, the presence of ciprofloxacin decreased greatly biofilm formation of P. aeruginosa N31. Such decrease was prominent at 1.9 μg/ml and above, a gradual decrease in biofilm forming capacity by dexpanthenol alone at the different concentrations used was detected. For isolates mixture N31, steep decrease in the biofilm forming capacity by all antibiotic concentrations alone or in presence of dexpanthenol. Dexpanthenol alone caused a marked decrease in the biofilm forming capacity (Figure 4a). Nearly, the same pattern was observed for S. aureus N6, isolate mixture N6 (Figure 4b) as well as the other isolates.
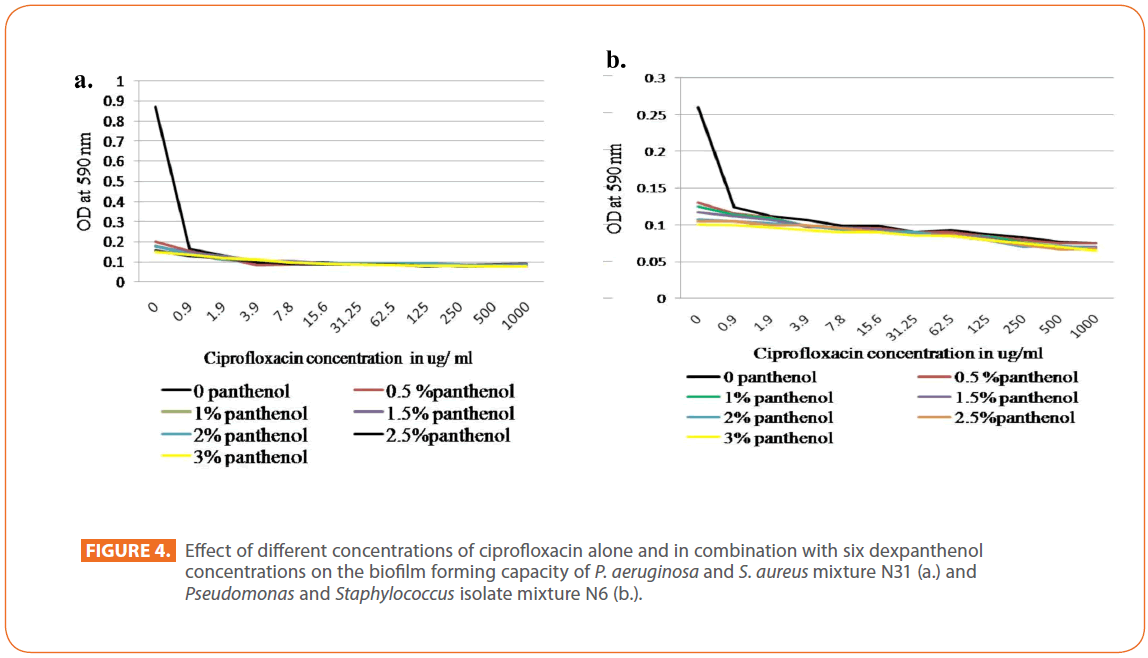
Figure 4: Effect of different concentrations of ciprofloxacin alone and in combination with six dexpanthenol concentrations on the biofilm forming capacity of P. aeruginosa and S. aureus mixture N31 (a.) and Pseudomonas and Staphylococcus isolate mixture N6 (b.).
For colistin sulphate, a marked decrease in biofilm forming capacity of P. aeruginosa N31was detected by all dexpanthenol concentrations alone, and in combination with colistin, while, a gradual decrease in biofilm forming capacity was noticed when colistin was applied alone. On the other hand, a marked decrease in the biofilm forming capacity of isolates mixture N31was noted in all dexpanthenol and colistin alone or in combination (Figure 5). For Pseudomonas isolate N4, great decrease in biofilm forming capacity by dexpanthenol alone was observed that increased gradually when combined with colistin. The colistin alone resulted in abrupt decease in biofilm forming capacity that increased gradually by increasing concentration. Regarding the effect of dexpanthenol on antibiofilm activity of the antibiotics, results were found to be statistically highly significant by Paired T test and CI =95% using MiniTab 16 especially in case of gentamicin and ciprofloxacin (P=0.001 to < 0.09).
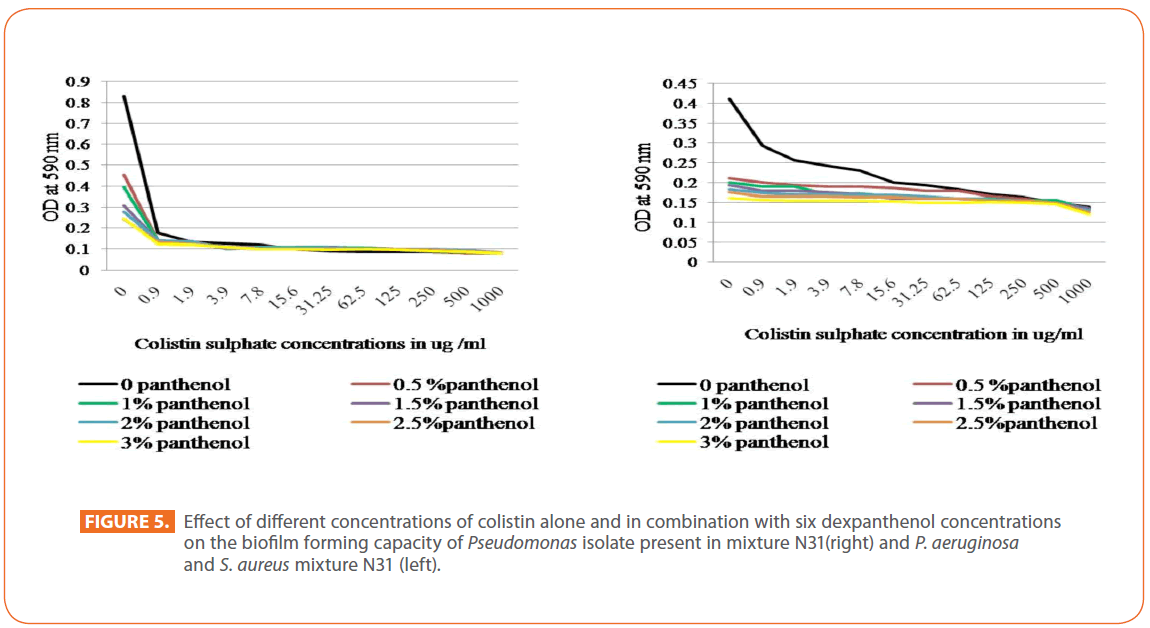
Figure 5: Effect of different concentrations of colistin alone and in combination with six dexpanthenol concentrations on the biofilm forming capacity of Pseudomonas isolate present in mixture N31(right) and P. aeruginosa and S. aureus mixture N31 (left).
Determination of efflux inhibition activity of dexpanthenol using ethidium bromide
A simple qualitative method was applied just to shed light on one of the proposed mechanisms for the dexpanthenol being able to convey synergistic effect with the tested antibiotics despite being without an antimicrobial activity of its own in the concentrations tested in the current study. For P. aeruginosa present in isolate mixture N31, an increase in the fluorescence intensity is observed in wells B2 (0.5% dexpanthenol) and C2 (3% dexpanthenol) as compare to A2 (No dexpanthenol). From left to right in rows B and C, the fluorescence emitted by the bacteria grown in decreasing concentration of EB is up to well B4(1μg/ml) and C5 (0.5μg/ml) while in absence of dexpanthenol is up to A3 (2 μg/ml ) (Figure 6A). For P. aeruginosa present in isolate mixture N6, in rows B and C, the fluorescence emitted by the bacteria grown in decreasing concentration of EB is up to well B4 (1μg/ml) and C4 (1μg/ml), while in absence of dexpanthenol is up to A3 (2μg/ml) (Figure 6B).
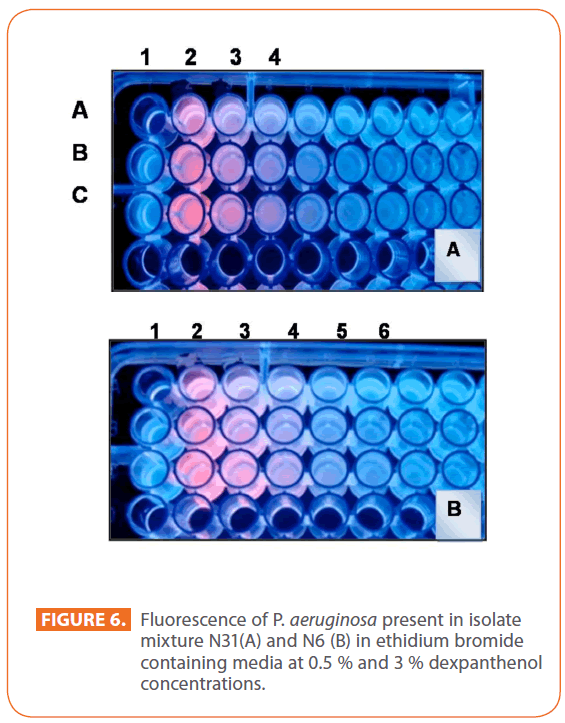
Figure 6: Fluorescence of P. aeruginosa present in isolate mixture N31(A) and N6 (B) in ethidium bromide containing media at 0.5 % and 3 % dexpanthenol concentrations.
Propolis antimicrobial activity
EEP showed antibacterial activity against S. aureus and P. aeruginosa isolates of N31 mixture (Figure 7).
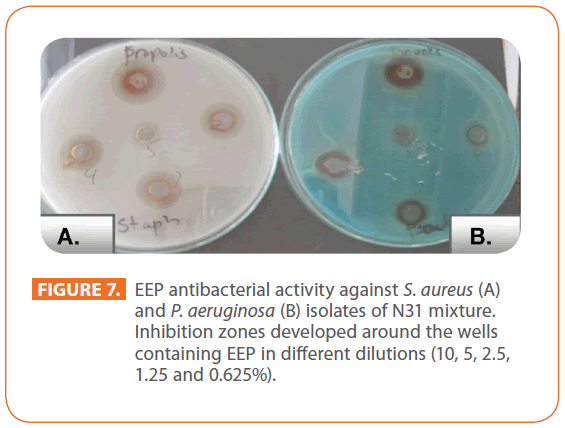
Figure 7: EEP antibacterial activity against S. aureus (A) and P. aeruginosa (B) isolates of N31 mixture. Inhibition zones developed around the wells containing EEP in different dilutions (10, 5, 2.5, 1.25 and 0.625%).
Determination of the MIC of EEP by agar dilution technique
Pseudomonas isolate in mixture N31 demonstrated the highest degree of resistance against EEP (MIC = 5000 μg/ml) while Pseudomonas N4 was the least resistant among the selected isolates (MIC = 625 μg/ml). The other MIC values for the remaining isolates were: 2500 μg/ml for Pseudomonas isolate N6 and Staphylococcus isolates N31 and N25 and 1250 μg/ml for Staphylococcus isolate N6.
Effect of EEP on the different antibiotic sensitivity pattern of the selected isolates
The effect of sub-inhibitory concentration (0.25MIC) of EEP on the zone of inhibition diameter of tested antibiotics applied against the selected isolates revealed a synergistic effect for most of antibiotics applied in case of S. aureus isolate N31. The organism which was resistant to fluoroquinolones, cephalosporins and imipenem became sensitive to antibiotics in presence of EEP compared to the tested antibiotics alone. For P. aeruginosa isolate N31, no effect was detected upon testing with the applied antibiotics (Figure 8). The same was observed for the other selected isolates.
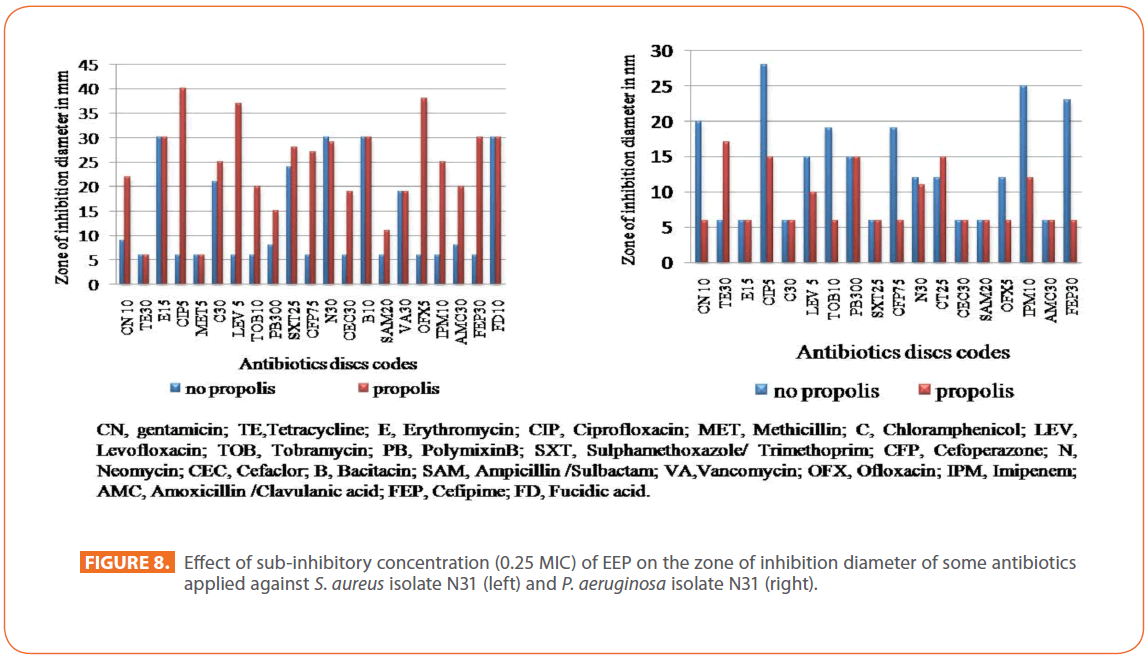
Figure 8: Effect of sub-inhibitory concentration (0.25 MIC) of EEP on the zone of inhibition diameter of some antibiotics applied against S. aureus isolate N31 (left) and P. aeruginosa isolate N31 (right).
Effect of EEP on the capacity of biofilm formation by the selected isolates
An abrupt decrease in the biofilm formation was observed for all isolates and mixtures being more markedly observed in case of isolates mixture N31 (Figure 9).
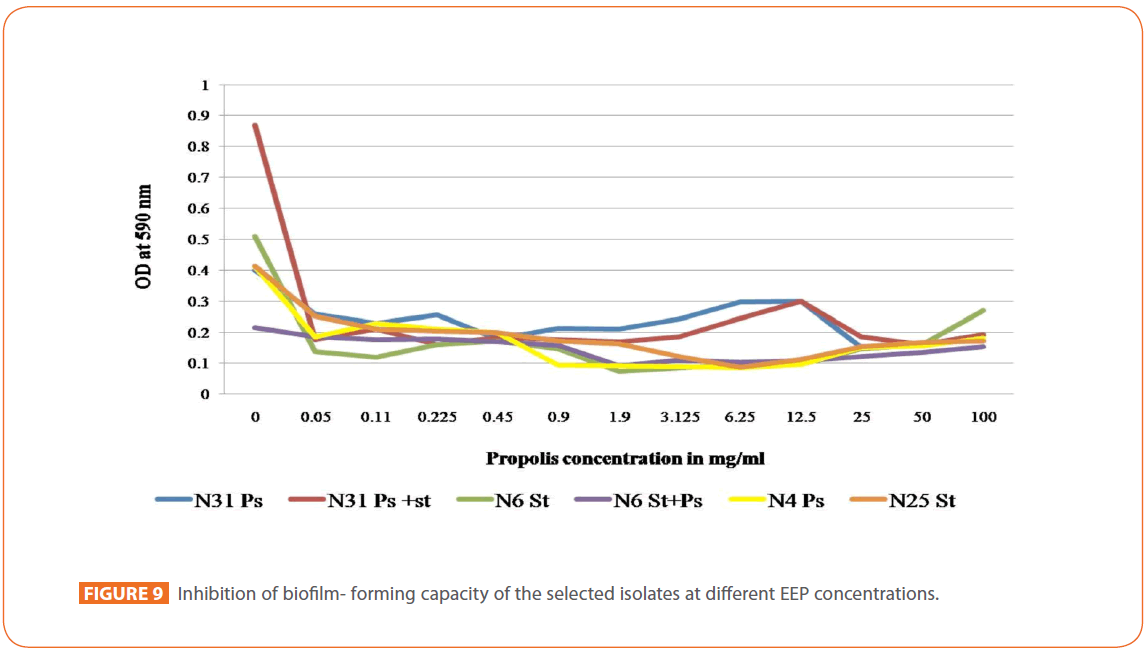
Figure 9: Inhibition of biofilm- forming capacity of the selected isolates at different EEP concentrations.
In vivo application of different gel formulation on induced wound infections in albino rat models
Figure 10 illustrates the images as well as the histopathological sections of representative rats, infected with P. aeruginosa and S. aureus N31 culture mates; one day after infection and when healing occurred upon using different treatment regimen. Figure 10 a. illustrates the control rat received no treatment showing no healing. Histopathological examination of the wound section (H&E, X400) reveals infected subcutaneous tissue with aggregates of basophilic grape-like clusters of S. aureus, and few rods of P. aeroginosa. The bacterial colonies are surrounded by prominent capillary proliferation with fibroblasts and infiltrated by neutrophils with cuffing of neutrophils around the capillaries (septic granulation tissue) associated with marked congestion. Degenerative muscular changes and fat necrosis are also noticed (hyalinization). Figure 10b. demonstrates group (1) rat received placebo carbopol gel 0.5% showing incomplete healing with scar formation. Histopathological examination (H&E, X200) shows that infection extends from the epidermis with gaping (abscess formation) and through the dermis to subcutaneous tissue. The inflammatory infiltrate is made up mainly of sheets of polymorphonuclear leukocytes and foamy histiocytes. Markedly congested proliferating capillaries are seen with hemorrhage and extension of inflammation to the skeletal muscle fibers. Figure 10c. demonstrates rat from group (2) treated with dexpanthenol 4% in 0.5% carbopol gel showing partial healing with scar formation. Histopahological examination (H&E, X400) shows granuloma formation made up mainly of collection of epithelioid histiocytes admixed with few neutrophils, lymphocytes and foamy histiocytes. The dermis and subcutaneous tissue show frequent mast cells. Prominent capillary proliferation is noted both in dermis and subcutaneous tissue.
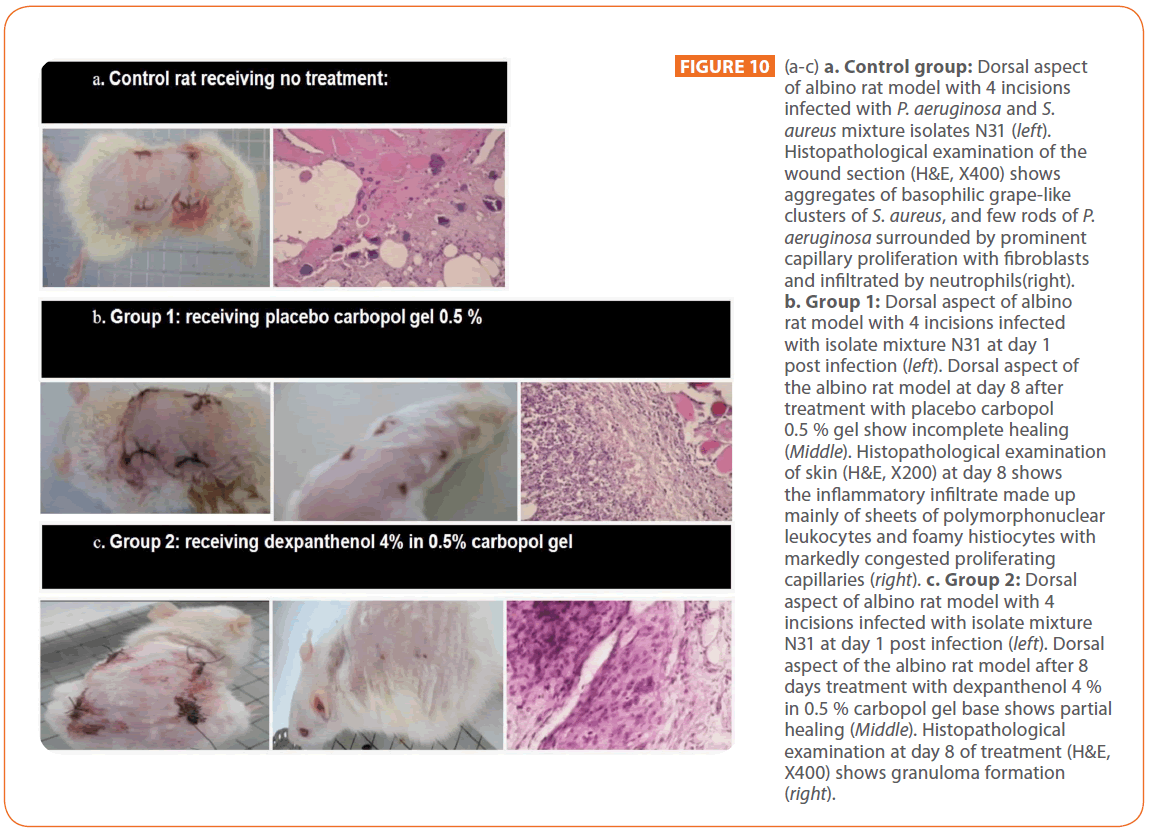
Figure 10: (a-c) a. Control group: Dorsal aspect of albino rat model with 4 incisions infected with P. aeruginosa and S. aureus mixture isolates N31 (left). Histopathological examination of the wound section (H&E, X400) shows aggregates of basophilic grape-like clusters of S. aureus, and few rods of P. aeruginosa surrounded by prominent capillary proliferation with fibroblasts and infiltrated by neutrophils(right). b. Group 1: Dorsal aspect of albino rat model with 4 incisions infected with isolate mixture N31 at day 1 post infection (left). Dorsal aspect of the albino rat model at day 8 after treatment with placebo carbopol 0.5 % gel show incomplete healing (Middle). Histopathological examination of skin (H&E, X200) at day 8 shows the inflammatory infiltrate made up mainly of sheets of polymorphonuclear leukocytes and foamy histiocytes with markedly congested proliferating capillaries (right). c. Group 2: Dorsal aspect of albino rat model with 4 incisions infected with isolate mixture N31 at day 1 post infection (left). Dorsal aspect of the albino rat model after 8 days treatment with dexpanthenol 4 % in 0.5 % carbopol gel base shows partial healing (Middle). Histopathological examination at day 8 of treatment (H&E, X400) shows granuloma formation (right).
Figure 10d. demonstrates a rat from group (3) treated by gentamicin 0.4 % in 0.5 % carbopol gel. A complete healing without scar was noticed. Histopathological examination (H&E, X200), showing a section involving the epidermis and the upper dermis, with complete resolution of the inflammation. Figure 10e. demonstrates a rat from group (4) treated with gentamicindexpanthenol gel; complete healing without scar was noticed. However, the application of dexpanthenol with gentamicin shortened the time taken for the wound to heal completely without scar as histopathological examination at day 6 treatment showed intact subcutaneous tissue and skeletal muscles with no sign of inflammation or infection. There is mild capillary congestion in subcutaneous tissue. Figure 10f. demonstrates a rat from group (5) treated with ciprofloxacin 0.2% in carbopol gel; a complete healing without scar was noticed. Histopathological examination shows intact epidermis, dermis & skeletal muscles with no evidence of inflammation. Figure 10g. demonstrating a rat from group (6) treated with ciprofloxacin 0.2 % and dexpanthenol 4 % in carbopol gel; complete scarless healing was noticed. However, the application of dexpanthenol with ciprofloxacin shortened the time taken for the wound to heal completely with no scar. Histopathological examination (H&E, X 400) demonstrates subcutaneous tissue showing mild chronic mononuclear inflammatory infiltrate.
Discussion
In order for wounds to heal normally, specific issues must be addressed such as aggressive debridement that allows the wound to move more rapidly from inflammation to proliferation minimizing the risk of infection. However, a regimen of topical antimicrobial agents should be considered to reduce an unacceptable level of bacteria and other pathogens. Adjunct microbial chemotherapy was also a field of investigation [24].
In the current work, the antimicrobial activity of dexpanthenol that was assessed against the selected isolates and mixtures revealed that dexpanthenol tested concentration has no antimicrobial activity. A previous study [25] confirmed the bacteriostatic properties of the dexpanthenol at MIC amounting to over 10% which is very high. In the present study, the effect of different dexpanthenol concentrations was tested on the MIC of four selected topically applied antibiotics including colistin sulphate. Colistin sulphate was chosen as an old antibiotic coming into action [16]. It has attracted more interest recently because of its significant activity that proved encouraging in comparison with previous experience [26].
In the present study, variable synergistic effects appeared as gradual or abrupt decrease in the MIC of the challenged antibiotics against the selected isolates at different dexpanthenol concentrations was noticed. This could be explained as pantothenic acid is essential for the growth of pathogenic microorganisms. Dexpanthenol is one of the analogues of pantothenic acid that compete with pantothenate. Dexpanthenol could be up-taken by bacteria instead of pantothenate leading to inhibition of fatty acid synthesis essential for cell wall lipid manufacturing resulting in a weak cell wall that increases passage of antibiotics. Spry et al. [27] stated that this could represent a potential target for the development of novel antibacterial therapeutics.
Currently, by comparing the MIC values of gentamicin against Pseudomonas N31 with that of the isolates mixture N31, a 4 fold increase of the MIC was observed. Such interesting result could be explained by the production of aminoglycoside acetyltransferase by Staphylococcus culture mate as stated by Martel et al. [28] leading to development of resistance to aminoglycosides. Therefore, the production of aminoglycosides modifying enzymes by Staphylococcus protected both Staphylococcus and Pseudomonas isolates from the effect of gentamicin.
Furthermore, in the present work, testing the prevention of biofilm formation using the selected antibiotics either alone or in combination with the six dexpanthenol concentrations revealed that gentamicin alone showed a great decrease in biofilm formation of Pseudomonas isolate N31 at 0.9 μg/ml, while original MIC was 250 μg/ml, meaning that in a range of sub-inhibitory concentration, the drug was able to prevent the adhesion of Pseudomonas single isolate and mixture N31 and therefore counteracting their pathogenicity. Similar finding reported for Staphylococcus isolate and mixture N6. The reduction in biofilm formation was also noticed when the other selected antibiotics were assayed against the selected isolates and mixtures. Inhibition of biofilm formation at subinhibitory concentrations of antimicrobial agents was reported previously [29-33].
In the present study, dexpanthenol by its own decreased greatly the adhesion of isolates and mixtures under test and assisted the antibiotic in its action in prevention of adhesion of the tested isolates. This capacity is thought to be due to the importance of pantothenic acid as a vitamin in the growth media in the procedure of biofilm formation and production of exopolysaccharides. As a result to structure similarity between dexpanthenol and pantothenic acid, the bacteria are tricked and the reaction stops and a decrease in the biofilm mass is the result as stated by Grobben et al. [34].
Biofilm-dwelling bacteria are particularly resistant to antibiotics, making it hard to eradicate. Bacteria rely on efflux pumps to get rid of toxic substances. Kvist et al. [35] discovered that efflux pumps are highly active in bacterial biofilms, thus making efflux pumps attractive targets for antibiofilm measures. Since many types of antibiotic resistance in bacteria are known to be conferred by efflux pumps, it is not surprising that enhanced efflux pump activity occurs concomitantly with enhanced antibiotic resistance. In line with this reasoning, interference with efflux pump activity during biofilm growth should be predicted to impact the biofilm-forming capacity. Werle [36] considered acyclic amines including dexpanthenol are drug efflux inhibitors. The production of N-acyl homoserine lactone- one of the signaling molecules mediate the biofilm formation- in medium is assisted by efflux pump in P. aeruginosa. Thus a decrease in biofilm formed mass and consequently reduction of invasiveness of the organism is associated with efflux pump inhibition [37]. From the present work, dexpanthenol could have mild efflux inhibition activity in case of Pseudomonas isolate N31 and to a lesser extent in case of Pseudomonas isolate N6. Therefore, it could affect the ability of the organism to extrude both the antibiotics and the N-acyl homoserine lactone, thus disturbing its ability to form biofilm. Interestingly, Pseudomonas isolate N6 produced a slightly higher N-acyl homoserine lactone compared to Pseudomonas isolate N31. Therefore, efflux pump inhibition could be a possible mechanism that needs further investigation putting into consideration the role played by dexpanthenol in weakening the cell wall.
Propolis or Bee glue is a newly introduced old remedy for treatment of wound and proved to have antibacterial action [10]. In the Egyptian market, propolis is stuck in its role as a dietary supplement; however, we tried here to shed light on its antimicrobial anti-biofilm efficacy. In present study, propolis demonstrates a slightly higher antibacterial action against Staphylococcus (Gram positive) than Pseudomonas (Gram negative) isolates. Our results were in accordance with previous reports [38,39]. Mechanisms of activity of propolis against microorganisms are still controversial. Some components present in propolis extracts like flavonoids and caffeic acid, benzoic acid, cinnamic acid, probably act on cell wall, causing functional and structural damages [40]. In the present work, EEP in sub-inhibitory concentration showed synergism with some antibiotics such as those interfere with bacterial protein synthesis and antagonism with others and this finding was in accordance with previous studies [22,41]. Oner et al. [12] confirm the safety and efficacy of intraarticular propolis and synergistic effect of propolis when used with cefazolin in an experimental septic arthritis model. Moreover, Arslan et al. [13] reported that Propolis was found to be effective on Candida albicans and Enterococcus faecalis, being more effective in lower concentrations on C. albicans than on E. faecalis. In the current study, EEP at low concentrations (0.05mg/ml) showed a drastic decrease in biofilm formation by Pseudomonas and Staphylococcus isolates and mixtures under test. Similar effect was detected by Scazzocchio et al. [42], as well; Kouidhi et al. [11] found that EEP possessed excellent protective effects against biofilms activity of oral streptococci.
Since the reduction of wound bioburden should reduce adverse influences that impede healing. Therefore, in vivo studies help clarify the role of bacterial biofilms in wound infection and healing. It also provides a tool to study antimicrobial efficacy of topical agents in the complex wound environment. In the present work, six groups, each of five rats were assayed using five different topical formulations and control placebo gel. Healing was considered achieved when scar-less complete healing of the induced infected wound occurred. Histopathological examination of the untreated wound section revealed infected subcutaneous tissue with aggregates of basophilic grape like clusters of S. aureus and few rods of Pseudomonas surrounded by prominent capillary proliferation; such effect matches to the colonization step cited by Kennedy et al. [43]. When carbopol gel alone was applied as placebo control, after 8 days, histopathology revealed extended infection through the skin layers with inflammation and congestion and these finding was matching with Smith et al. [44] who tested the role of acyl homoserine lactones (AHLs) in vivo as after injection into the skin of mice, they found a significant influx of white blood cells and subsequent tissue destruction. Therefore, the quorum- sensing systems of P. aeruginosa contribute to its pathogenesis both by regulating expression of virulence factors and by inducing inflammation. The marked congestion revealed histopathologically in non treated rats and those treated with only dexpanthenol gel is matching the inflammatory response induced by the presence of AHL as reported by Lawrence et al. [45].Yet, application of dexpanthenol with either gentamicin or ciprofloxacin shortened the time taken for the wound to heal completely without scar formation.
Conclusion
Consequently, we can conclude that, antibacterial action of the topically applied antibiotics is improved greatly in presence of dexpanthenol. It could be formulated in the same vehicle and administered locally to assist in prevention of wound infections putting into consideration the dexpanthenol antibiofilm activity, favoring healing in a short time. However, further investigations is needed about the mechanisms by which dexpanthenol could prevent biofilm formation. Propolis extract proved here its effective in vitro antimicrobial- antibiofilm activities; however, further in vivo study for propolis alone and in combination with antibiotics is essential as it could be of benefit in local wound management. Therefore, dexpanthenol and propolis alone or in combination with topically applied antibiotics proved effective in prevention of biofilm formation and hence could have a great impact in wound management.
200
References
- Fux CA, Costerton JW, Stewart PS, Stoodley P (2005) Survival strategies of infectious biofilms. Trends Microbiol 13:34–40.
- Edwards R, Harding KG (2004) Bacteria and wound healing. Curr Opin Infects Dis 17:91–96.
- James GA, Swogger E, Wolcott R, Pulcini E, Secor P, et al. (2008) Biofilms in chronic wounds. Wound Repair Regen 16:37–44.
- Gonzalez JE, Keshavan ND (2006) Messing with Bacterial Quorum Sensing. MicrobiolMolBiol Rev 70: 859–875.
- Chaignon P, Sadovskaya I, RagunahCh, Ramasubbu N, Kaplan JB, et al. (2007) Susceptibility of Staphylococcal biofilms to enzymatic treatments depends on their chemical composition. ApplMicrobiolBiotechnol 75: 125–132.
- Brackman G, Cos P, Maes L, Nelis HJ , Coenye T (2011) Quorum sensing inhibitors increase the susceptibility of bacterial biofilms to antibiotics in vitro and in vivo. Antimicrob Agents Chemother 55:2655-2661.
- Ebner F, Heller A, Rippke F, Tausch I (2002) Topical use of dexpanthenol in skin disorders. Am J ClinDermatol 3: 427–433.
- Bankova VS, de Castro SL, Marcucci MC (2000) Propolis: recent advances in chemistry and plant origin. Apidologie 31: 3–15.
- Drago L, Mombelli B, De Vecchi E, Fassina MC, Tocalli L, et al. (2000) In vitro antimicrobial activity of propolis dry extract. J Chemother 12: 390–395.
- McLennan SV, Bonner J, Milne S, Lo L, Charlton A, et al. (2008) The anti-inflammatory agent Própolis improves wound healing in a rodent model of experimental diabetes. Wound Repair Regen 16:706–713.
- Kouidhi B, Zmantar T, Bakhrouf A (2010) Anti-cariogenic and anti-biofilms activity of Tunisian propolis extract and its potential protective effect against cancer cells proliferation. Anaerobe 16:566-571.
- Oner M, Kafadar I, Guney A, Halici M, Deniz K, et al. (2011) Effect of intraarticularpropolis in an experimental septic arthritis model. J PediatrOrthop B 20:8-13
- Arslan S, Ozbilge H, Kaya EG, Er O (2011) In vitro antimicrobial activity of propolis, BioPure MTAD, sodium hypochlorite, and chlorhexidine on Enterococcus faecalisand Candida albicans. Saudi Med J 32:479-483.
- El-Banna TE, Abd El-Aziz AA, Sonbol FI, Helaly GF, Louise NL (2011) Detection of biofilms and their interaction in wound infection: Role of N-acyl homoserine lactone and other virulence factors in enhancement of biofilm formation. Egypt J Med lab sci 20:1-14
- Stepanovic S, Vukovic D, Dakic I, Savic B, Svabic-Vlahovic, M (2000) A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175- 179.
- Li J, Nation RL, Turnidge JD, Milne R W, Coulthard K, et al. (2006) Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6: 589–601.
- Hassan M A, Mohammed F A, Sabour E A (2003). Formulation and evaluation of ciprofloxacin hydrochloride and norfloxacin topical gel. S T P PharmaSci 13: 195-201
- Pillai SK, Moellering RC Jr, Eliopoulos GM (2005) Antimicrobial combinations. In :Lorian V editor. Antibiotics in laboratory medicine. Baltimore: Williams and Wilkins. pp. 365–441.
- Martins A, Spengler G, Rodrigues L, Viveiros M, Ramos J, et al. (2009) pH Modulation of Efflux Pump Activity of Multi-Drug Resistant Escherichia coli: Protection During Its Passage and Eventual Colonization of the Colon. PLoS ONE 4(8): e6656.
- Johnson K S , Eischen FA , Giannasi D E (1994) Chemical composition of North American bee propolis and biological activity towards larvae of greater wax moth (Lepidoptera: Pyralidae) J ChemEcol 20: 1783-1791.
- Stepanovi? S, Anti? N, Daki? I, Svabi?-Vlahovi? M (2003) In vitro antimicrobial activity of propolis and synergism between propolis and antimicrobial drugs. Microbiol Res 158: 353–357.
- Senthil Kumar M, Sripriya R, VijayaRaghavan H, SehgalPK(2006) Wound healing potential of Cassia fistula on infected albino rat model. J Surg Res 131 : 283–289.
- Klöcker N, Rudolph P, Verse T (2004) Evaluation of protective and therapeutic effects of dexpanthenol on nasal decongestants and preservatives: results of cytotoxic studies in vitro. Am J Rhinol 18:315-20.
- Barrett JF (2005) Adjunct microbial therapy-prospects for the future. Therapy 2: 67–76
- Tritsmans E, VanbenedenF(1956) Influence in vitro of panthenol on the resistance of staphylococci to antibiotics and sulfonamides. Antonie Van Leeuwenhoek 22: 273–280.
- Cai Y, Wang R, Liang BB, An MM (2009) In-vitro bactericidal activity of colistin against biofilm-associated Pseudomonas aeruginosaand Acinetobacterbaumannii. J HospInfec 72: 368– 370.
- Spry C, Kirk K, SalibaKJ(2008) Coenzyme A biosynthesis: an antimicrobial drug target. FEMS Microbiol Rev 32: 56–106.
- Martel A, Masson M, Moreau N, Le Goffic F (1983). Kinetic studies of aminoglycoside acetyltransferase and phosphotransferase from Staphylococcus aureusRPAL. Relationship between the two activities. Eur J Biochem133 : 515–521
- Yassien M, Khardori N, Ahmedy A, Toama M (1995) Modulation of biofilms of Pseudomonas aeruginosaby quinolones. Antimicrob. Agents Chemother 39: 2262–2268.
- Owlia P, Behzadiyan-Nejad Q, Souri E (2001) Microscopic study of the effects of sub-inhibitory concentrations of gentamicin on capsule production of Pseudomonas aeruginosa. Arch Iran Med 4: 18–20.
- Wojnicz D K?ak M, Adamski R, Jankowski S. (2007) Influence of sub-inhibitory concentrations of amikacin and ciprofloxacin on morphology and adherence ability of uropathogenic strains. Folia Microbiol 52: 429–436.
- Majtán J, Majtánová L, Xu M, Majtán V(2008) In vitro effect of sub-inhibitory concentrations of antibiotics on biofilm formation by clinical strains of Salmonella entericaserovartyphimurium isolated in Slovakia. J App Microbiol 104: 1294–1301.
- Liaqat I, Sumbal F, Sabri AN (2009) Tetracycline and chloramphenicol efficiency against selected biofilm forming bacteria. Curr Microbiol 59: 212–20.
- Grobben GJ, Chin-Joe I, Kitzen VA, Boels IC, Boer F, et al. (1998) Enhancement of exopolysaccharide production by Lactobacillus delbrueckiisubsp. bulgaricus NCFB 2772 with a simplified defined medium. Appl Environ Microbiol 64: 1333–1337.
- Kvist M, Hancock V, KlemmP(2008) Inactivation of efflux pumps abolishes bacterial biofilm formation. Appl Environ Microbiol 74: 7376–7382.
- WerleM(2008) Natural and synthetic polymers as inhibitors of drug efflux pumps. Pharm Res 25: 500–511.
- Hirakata Y, Kondo A, Hoshino K , Yano H, Arai K, et al. (2009) Efflux pump inhibitors reduce the invasiveness of Pseudomonas aeruginosa. Int J Antimicrob Agents 34: 343–346.
- FernandesJúnior A, Balestrin EC, Betoni JE, OrsiRde O, da Cunha Mde L, Montelli AC(2005) Propolis: anti-Staphylococcus aureusactivity and synergism with antimicrobial drugs. MemInstOswaldo Cruz 100: 563–566.
- Gonsales GZ, Orsi RO, FernandesJúnior A, Rodrigues P, FunariSRC(2006) Antibacterial activity of propolis collected in different regions of Brazil. J Venom Anim Toxins incl Trop Dis 12:276–284.
- Takaisi-Kikuni NB, Schilcher H (1994). Electron microscopic and microcalorimetric investigations of the possible mechanism of the antibacterial action of a defined propolis provenance. Planta Med 60: 222–227.
- Speciale A Costanzo R, Puglisi S ,Musumeci R, Catania MR, et al. (2006) Antibacterial activity of propolis and its active principles alone and in combination with macrolides, beta-lactams and fluoroquinolones against microorganisms responsible for respiratory infections. J Chemother 18: 164–171.
- Scazzocchio F, D’Auria FD, Alessandrini D, Pantanella F (2006) Multifactorial aspects of antimicrobial activity of Propolis. Microbiol Res 161: 327–333.
- Kennedy P, Brammah S, Wills E (2010) Burns, biofilm and a new appraisal of burn wound sepsis. Burns 36: 49–56.
- Smith RS, Harris SG, Phipps R, Iglewski B (2002) The Pseudomonas aeruginosaquorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J Bacteriol 184: 1132–1139.
- Lawrence RN, Dunn WR, Bycroft B, Camara M, Chhabra S R, et al. (1999) The Pseudomonas aeruginosaquorum-sensing signal molecule, N-(3-oxododecanoyl)-L-homoserine lactone, inhibits porcine arterial smooth muscle contraction. Br J Pharmacol. 128: 845–848.

















