Introduction
Giardia lamblia and Cryptosporidium parvum are both common enteric parasites in human causing gastrointestinal infections [1-3], manifested with a wide range of clinical spectrum ranging from asymptomatic carrier to long-lasting diarrhea with malabsorption [4-6]. Both parasites are transmitted through fecal-oral routes [7, 8], by consumption of contaminated food [9, 10] or water [3, 11]. In developing countries, crowded urban areas, poor water quality and a lack of sanitation enhance the risk of transmission [3, 12].
Contraction of the diseases is more frequent among younger children especially those in day care centers [13, 14]. These infections in childhood are of major importance as being the most frequent cause of malabsorptive diarrhea that have a negative impact on growth and development of children [15-17].
Conventional microscopical diagnosis of Giardia and Cryptosporidium infections is time-consuming, and relies crucially on the microscopist’s skills and experience [18]. Rapid antigen detection assays have the advantage of being more precise, rapid, simple and cost-effective modality, offering a relevant alternative method to the routine examination method and provide the added sensitivity required to confirm infections in patients with low parasite numbers [19].
The purpose of this study is to evaluate the diagnostic performance of an immunochromatographic (IC) test, ImmunoCard STAT! Crypt/Giardia (Meridian Bioscience, Inc.) for diagnosing giardiasis and cryptosporidiosis by comparing it with composite reference standard (CRS) of wet mount microscopy and ELISA copro-antigen assay.
Material and Methods
The study was carried out on a sample of 122 children attending the Outpatient Clinics at Mansoura University Children Hospital. Recruited children were in the age of 6 months to 14 years, suffering from persistent and/or recurrent diarrhea with the exclusion of children who had received anti-parasite treatment in the last three months before specimen collection. Diarrhea was defined as an increase of stool frequency to three or more times a day and/or the change of stool consistency into more liquid form.
Fresh stool specimens were collected in sterile leak proof containers and send immediately to laboratory. Each specimen was divided into three parts. The first part was left fresh for wet mount microscopy using formalin-ethyl acetate concentration method and mounted smears were stained by Trichrome [18] for detecting Giardia and Kinyoun modified acid-fast and Auramine-Rhodamine stains for detecting Cryptosporidium [20].
The second part was preserved in 10% formalin for performing the rapid IC ImmunoCard STAT! Crypt/Giardia assay according to the manufacturer’s instructions (Meridian Bioscience, Inc.). The assay procedure involves the addition of 2 drops of sample treatment buffer to a tube with 60μL of stool specimen then adding 2 drops of colloidal carbon-conjugated detection reagent for Giardia and Cryptosporidium. After mixing, the sample was immediately poured into the test device. Results were visualized after 10 min. positive results were visualized as gray-black lines. A positive control line was visible on the device each time the test was completed successfully.
The last third part of each stool specimen was immediately frozen and stored at -20°C. Subsequently, the frozen part were thawed and mixed thoroughly before testing for Giardia and Cryptosporidium copro-antigen using Ridascreen Giardia and Ridascreen Cryptosporidium (R-Biopharm) enzyme immunoassay respectively. Both tests were performed according to the manufacturers’ instructions.
Analysis
The validation parameters of the rapid IC assay were determined compared to those of CRS of wet mount microscopy and copro-antigen assay. Specimen was labeled as positive if the results of either wet mount or copro-antigen were positive. On the other hands, Specimen was labeled negative if both mount preparations and copro-antigen were negative. Data entry and analyses were performed using the SPSS statistical package version 16 (SPSS, Inc., Chicago, IL, USA). The description of qualitative data was done in form frequency and proportions. The chi-square (χ2) test was used to test for the potential association between groups for qualitative data. A p value of <0.05 was considered a significant difference at confidence interval 95%.
Result
Of 122 stool samples, 45 were confirmed as true positives for Giardia, and 9 for Cryptosporidium reporting 38.5% and 7.4% incidence of Giardia and Cryptosporidium in children suffering from chronic diarrheal illness, respectively. The results obtained with the different methods are shown in Table 1. It was noted that any positive sample by any method was also positive by Ridascreen Giardia enzyme immunoassay for diagnosis of Giardia and Kinyoun modified acid-fast for diagnosis of Cryptosporidium. So, the number of positive samples for Ridascreen Giardia enzyme immunoassay and Kinyoun modified acid-fast will represent the total number of positives for Giardia and Cryptosporidium, respectively.
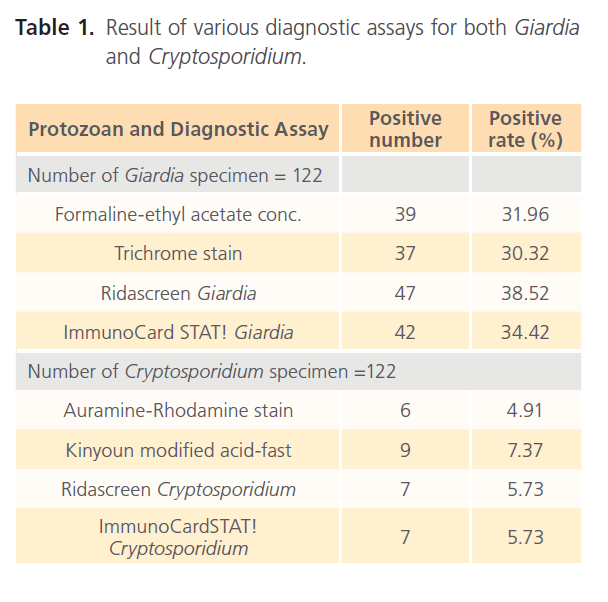
Table 1: Result of various diagnostic assays for both Giardia and Cryptosporidium.
In validating the immunochromatographic ImmunoCard STAT! Crypt/Giardia assay against the reference methods, the calculated sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPP) and diagnostic accuracy (DA) were 89, 100, 100, 94% and 96% for Giardia and 78, 100, 100, 98 and 98% for Cryptosporidium, respectively (Table 2). There were 5 false negative specimen for giardiasis and 2 false negative cryptosporidiosis recorded by ImmunoCard STAT! Crypt/Giardia assay.
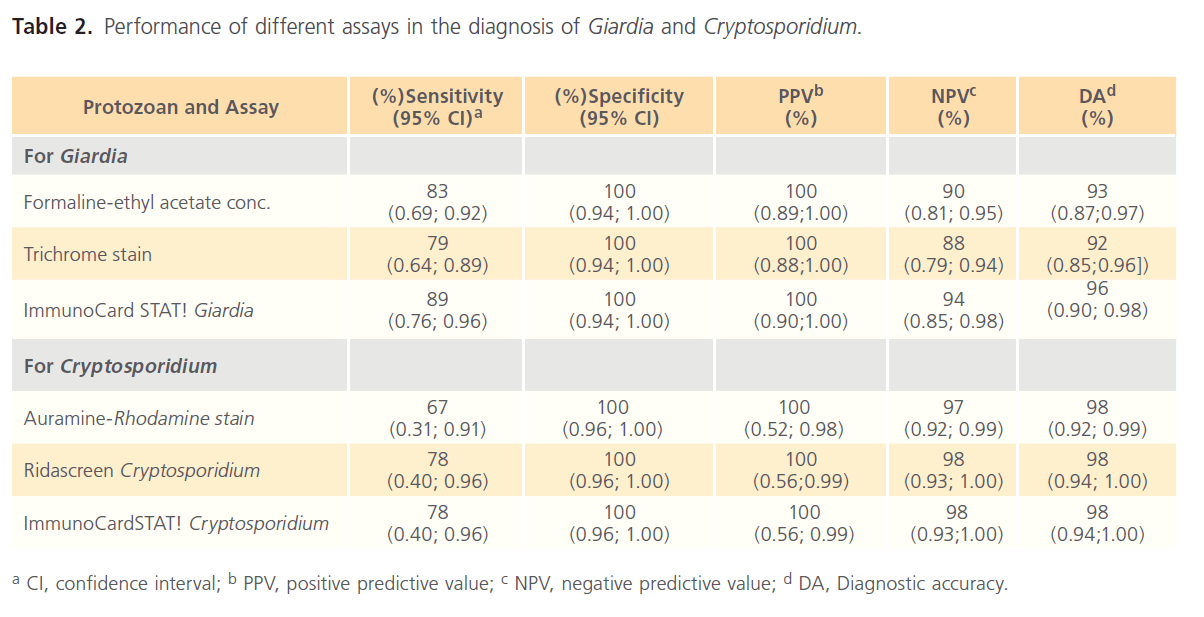
Table 2: Performance of different assays in the diagnosis of Giardia and Cryptosporidium.
Discussion
The detection of Giardia and Cryptosporidium antigens in stool specimens using enzyme immunoassays has become an accepted approach to diagnosis but, it’s laborious, timeconsuming and can be used in epidemiological survey for screening multiple specimens simultaneously but not as a routine laboratory technique [21]. So, there is need for better diagnostic method that surpasses the limitations of the currently available techniques and not to miss any case of infection. In this context, the immunochromatographic rapid test was tried and its performance was assessed in reference to other conventional methods.
In this study, the overall incidence of both Giardia and Cryptosporidium in children with diarrheal illness was 38.5% and 7.4% respectively. A similar study done in Cairo [22] reported 31.1% incidence for Giardia and 19.8% for Cryptosporidium, this difference may be attributed to the different epidemiological patterns of parasitic infection in Egypt.
The performance of ImmunoCardSTAT! Cryptosporidium against the CRS was 78, 100, 100, 98 and 98% for sensitivity, specificity, PPV, NPV and DA respectively. The sensitivity of the ImmunoCardSTAT! was less than that of Kinyoun modified acid-fast, equal to Ridascreen immunoassay and more than Auramine-Rhodamine stain. The ImmunoCard STAT! Cryptosporidium assay may eliminate some of the skill needed in performing complicated staining procedures and recognizing the morphology of the small oocyst. However, staining holds importance due to its low cost in addition to having a comparable efficacy with the assay [23].
A recent study [24] conducted in Egypt to evaluate the ImmunoCard STAT! Cryptosporidium assay on 315 immunocompromised patients, have reported 96% (95% CI, 87% to 104%) sensitivity and 97% (95% CI, 93-103) as the total accuracy of the assay. Another study [19] reported 98.8% sensitivity, 100% specificity, 100% PPV, and 99.7% for NPV for the same assay using a total of 401 specimens. On the other hand, a similar study [25] carried on 246 specimens reported 67.6% sensitivity, 99% specificity for ImmunoCardSTAT! Cryptosporidium. The difference in sensitivities may be due to diversity of genotypes of Cryptosporidium among different population, the immune status of patients and also due to the different number of samples used in each study.
In the current study, 2 false negative specimens for Cryptosporidium were reported by the ImmunoCard STAT! , this may be because both ImmunoCardSTAT! and Ridascreen immunoassay detect only Cryptosporidium parvum [26] while, human Cryptosporidiosis is generally caused by the species Cryptosporidium hominis beside many different genotypes and subtypes of Cryptosporidium [14]. Also, both assays generally detect positive samples with >175 organisms/10 µl, they often fail to detect samples with small numbers of parasites that can be detected by staining more than one smear of same sample [25].
Regarding giardiaiasis, Ridascreen Giardia immunoassay proved to be the most sensitive method in the diagnosis of Giardiasis. In validating ImmunoCard STAT! Giardia against that CRS, 89% sensitivity, 100% specificity, 100% PPV and 94% NPV were recorded. In this study, ImmunoCard STAT! Giardia proved to be more sensitive than both Formalin-ethyl acetate concentration and Trichrome staining.
It was reported that the SIMPLE-READ Giardia rapid assay (Medical Chemical Corp.), which can also be used at the point of service like other rapid immunoassays currently available, have 97.2% sensitivity and 100% specificity, the PPV (predictive value of a positive test result) was 100%, and the NPV (predictive value of a negative test result) was 97.2% [27].
Using the results of the individual ELISAs as gold standards, the Giardia/Cryptosporidium Check assay had a sensitivity of 98.4%, 100% specificity, a positive predictive value of 98.7%, and a negative predictive value of 99.3%. ImmunoCard STAT! appears to have comparable sensitivities and specificities to those of the Giardia/Cryptosporidium Chek test [28, 29].
The limitation of the study was the relatively high cost of ImmunoCard STAT! Crypt/Giardia test and also, the limited number of specimens as only children suffering from chronic diarrhea illness not taking anti-parasitic treatment since three months were incorporated in the study.
Conclusion
The rapid ImmunoCard STAT! Crypto/Giardia immunochromatographic assay is simple to perform, objective, and timesaving. It can be used in integration with other diagnostic modality to confirm diagnosis or used as a rapid service assay when other methods are unavailable especially in waterborne outbreak situation. However, it’s important to search for another available alternative with high degree of accuracy, sensitivity, and specificity and keeping both time and cost low.
160
References
- O’Donoghue PJ (1995) Cryptosporidium and cryptosporidiosis in man and animals. Int J Parasitol 25:139–195
- Adam RD (2001) Biology of Giardia lamblia. ClinMicrobiol Rev 14(3):447-475 https://www.ncbi.nlm.nih.gov/ pubmed?term=Adam%20%5Bau%5D%20and%202001%20 %5Bdp%5D%20and%20giardia
- Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol 22(5):203- 208 https://www.ncbi.nlm.nih.gov/pubmed?term=Savioli%20 %5Bau%5D%20and%202006%5Bdp%5D%20and%20giardia
- Chen XM, Keithly JS, Paya CV, LaRusso NF (2002) Cryptosporidiosis.NEngl J Med 346: 1723–1731
- Yadollahie M, Roshanipoor M, Motallebipoor SA, Habibzadeh F (2002) Giardiasis in a 16-day-old neonate. East Mediterr Health J 8(1):189-191
- Gatei W, Wamae CN, Mbae C, Waruru A, Mulinge E, Waithera T, Gatika SM, Kamwati SK, Revathi G, Hart CA (2006) Cryptosporidiosis: prevalence, genotype analysis, and symptoms associated with infections in children in Kenya. Am J Trop Med Hyg 75(1):78-82 https:// www.ncbi.nlm.nih.gov/pubmed/16837712
- Smith, H V, Caccio SM, Tait A, McLauchlin J, Thompson ARC (2006) Tools for investigating the environmental transmission of Cryptosporidium and Giardia. Trends Parasitol 22:160–167
- Fayer R, Fair PA, Bossart GD, Santin M (2008) Examination of naturally exposed bottlenose dolphins (Tursiopstruncatus) for Microsporidia, Cryptosporidium and Giardia. J Parasitol 94:143–147 https://www.ncbi. nlm.nih.gov/pubmed/18372633
- Quiroz ES, Bern C, MacArthur JR, Xiao L, Fletcher M, Arrowood MJ, Shay DK, Levy ME, Glass RI, Lal A (2000) An outbreak of cryptosporidiosis linked to a foodhandler. J Infect Dis 181(2):695- 700 https://www.ncbi.nlm.nih.gov/pubmed?term=Quiroz%20%20 %5Bau%5D%20and%202000%20%5Bdp%5D%20and%20 cryptosporidiosis
- Olsen SJ, MacKinnon LC, Goulding JS, Bean NH, Slutsker L (2000) Surveillance for foodborne disease outbreaks United States, 1993– 1997. Morb Mortal Wkly Rep 49:1–62. https://www.ncbi.nlm.nih.gov/ pubmed/10789699
- Hellard M, Hocking J, Willis J, Dore G, Fairley C (2003) Risk factors leading to Cryptosporidium infection in men who have sex with men. Sex Transm Infect 79:412–414 https://www.ncbi.nlm. nih.gov/pubmed?term=Hellard%20%5Bau%5D%20and%20 2003%5Bdp%5D%20and%20cryptosporidium
- Karanis P, Kourenti K, Smith HV (2007) Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J Water Health 5:1–38
- Núñez FA, González OM, Bravo JR, Escobedo AA, Gonzaléz I (2003) Intestinal parasitosis in children admitted to the Pediatric Teaching Hospital of Cerro, Havana City, Cuba. Rev Cubana Med Trop 55(1):19- 26.
- Xiao L, Fayer R, Ryan U, Upton SJ (2004) Cryptosporidium taxonomy:recent advances and implications for public health. ClinMicrobiol Rev 17:72–97 15.
- Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM (2002) Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 16;359(9306):564-571
- Simsek Z, Zeyrek FY, Kurcer MA (2004) Effect on Giardia infection on growth and psychomotor development of children aged 0–5 years. J Trop Pediatr 50:90–93. https://www.ncbi.nlm.nih.gov/ pubmed/15088797
- Ayuo PO (2009) Human cryptosporidiosis: a review. East Afr Med J 86(2):89-93
- Garcia LS (2001) Diagnostic medical parasitology, 4th edn. ASM Press,Washington, D.C.
- Garcia LS, Shimizu RY, Novak S, Carroll M,Chan F (2003) Commercial assay for detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens by rapid solid-phase qualitative immunochromatography. J ClinMicrobiol 41:209–212 https://www. ncbi.nlm.nih.gov/pubmed/12517850
- Garcia LS, Bruckner DA, Brewer TC, Shimizu RY(1983) Techniques for the recovery and identification of Cryptosporidium oocysts from stool specimens. J ClinMicrobiol 18: 185–190 https://www.ncbi. nlm.nih.gov/pubmed?term=Garcia%20%5Bau%5D%20and%20 1983%5Bdp%5D%20and%20cryptosporidium
- Al-Saeed AT, Issa SH (2010) Detection of Giardia lamblia antigen in stool specimens using enzyme-linked immunosorbent assay. East Mediterr Health J 16(4):362-364 https://www.ncbi.nlm.nih.gov/ pubmed?term=Al-Saeed%20%5Bau%5D%20and%202010%20 %5Bdp%5D%20and%20giardia
- Mousa KM, Abdel-Tawab AH, Khalil HH, El-Hussieny NA (2010) Diarrhea due to parasites particularly Cryptosporidium parvum in great Cairo, Egypt. J Egypt SocParasitol 40(2):439-450 https://www.ncbi. nlm.nih.gov/pubmed?term=Mousa%20%5Bau%5D%20and%20 2010%5Bdp%5D%20and%20cryptosporidium
- Tuli L, Singh DK, Gulati AK, Sundar S, Mohapatra TM (2010) A multiattribute utility evaluation of different methods for the detection of enteric protozoa causing diarrhea in AIDS patients. BMC Microbiol 15;10:11 https://www.ncbi.nlm.nih.gov/pubmed?term=Tuli%20 %5Bau%5D%20and%202010%20%5Bdp%5D%20and%20 protozoa
- El-Moamly AA, El-Sweify MA (2011) ImmunoCard STAT! cartridge antigen detection assay compared to microplate enzyme immunoassay and modified Kinyoun’s acid-fast staining technique for detection of Cryptosporidium in fecal specimens. Parasitol Res. In press. https:// www.ncbi.nlm.nih.gov/pubmed?term=ImmunoCard%20STAT!%20 cartridge%20antigen%20detection%20assay%20compared%20 to%20microplate%20enzyme%20immunoassay%20and%20 modified%20Kinyoun’s%20acid-fast%20staining%20technique%20 for%20detection%20of%20Cryptosporidium%20in%20fecal%20 specimens
- Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP (2003) Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J ClinMicrobiol 41: 623–626 https://www.ncbi.nlm.nih.gov/pubmed?term=Johnston%20 %5Bau%5D%20and%202003%5Bdp%5D%20and%20giardia
- Molloy SF, Smith HV, Kirwan P, Nichols RA, Asaolu SO, Connelly L, Holland CV (2010) Identification of a high diversity of Cryptosporidium species genotypes and subtypes in a pediatric population in Nigeria. Am J Trop Med Hyg 82(4):608-613 https://www.ncbi.nlm.nih.gov/ pubmed/20348508
- Garcia LS, Garcia JP (2006) Detection of Giardia lamblia antigens in human fecal specimens by a solid-phase qualitative immunochromatographic assay. J ClinMicrobiol 44:4587–4588 https:// www.ncbi.nlm.nih.gov/pubmed/17065273
- Garcia, LS, Shimizu RY (1997) Evaluation of nine immunoassay kits(enzyme immunoassay and direct fluorescence) for the detection of Giardia lamblia and Cryptosporidium parvum in human fecal specimens. J ClinMicrobiol 35:1526–1529 https://www.ncbi.nlm.nih. gov/pubmed?term=Garcia%20%5Bau%5D%20and%201997%20 %5Bdp%5D%20and%20giardia
- Garcia, LS, Shimizu RY (2000) Detection of Giardia lamblia and Cryptosporidium parvum antigens in human fecal specimens using the ColorPAC combination rapid solid-phase qualitative immunochromatographic assay. J ClinMicrobiol







