Zhang Wenping1#, Qi Lixin2#, Le Wei2, Hu Jiahua3, Lihe Guo3, Huai L Feng4 and Zhang Jinfu5*
1Department of Dermatology, Tongji Hospital, Tongji University School of Medicine, Shanghai, P.R. China
2Department of Urology, Tongji Hospital, Tongji University School of Medicine, Shanghai, P.R. China
3Department of Molecular Cell Biology, Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Shanghai, P.R. China
4Department of Obstetrics and Gynecology, New York Hospital Queens affiliated Weill Medical College of Cornell University, New York, USA
5Department of Urology, Shanghai Tongren Hospital, Shanghai, P.R. China
#Equal Contribution
*Corresponding Author:
Zhang Jinfu
Department of Urology, Shanghai Tongren Hospital
1111 Xianxia Road, Shanghai, 200336, P.R. China
Tel: 086-21-62909915
E-mail: zhangjinfu@tongji.edu.cn
Received Date: October 28, 2017; Accepted Date: November 15, 2017; Published Date: November 20, 2017
Citation: Wenping Z, Lixin Q, Wei L, Jiahua H, Gua L, et al. (2017) Differential Distribution of GABA and GAT1 in Mouse Epididymis. Ann Clin Lab Res Vol.5: No.4:203. DOI: 10.21767/2386-5180.1000203
Keywords
Immunohistochemistry; Epididymis; GABA; GAT1
Abbreviations
GABA: γ-Aminobutyric Acid; GAT1: GABA Transport Protein I
Introduction
γ-aminobutyric acid (GABA) is a main inhibitory neurotransmitter in the mammalian central nervous system [1]. Neuronal inhibition is induced following GABA binding with ionic GABAA/GABAC receptor and metabolic GABAB receptor of postsynaptic membrane. GAT1 is a primary neuron transporter in brain among the four GATs (GAT1-GAT4) [2,3]. Synaptic transmission is terminated by GABA transporter protein (GAT) reuptaking GABA. In addition to localization in CNS, GABA is also identified in peripheral tissues [4], such as endocrine organs (pituitary, liver and ovary). It has been published previously that GABA is also presented in testis, sperm, deferent duct and epididymis in male reproductive system [5]. However the distribution of GABA and GAT1 in epididymis is still not clear. Similar to progesterone, GABA causes depolarization of cell membrane and stimulates acrosome reactions in recapacitated human spermatozoa in a dose dependent manner [6]. GABAA and GABAB receptors, expressed in sperms, might relate to acrosome reaction via GABA and progesterone [7-11]. GABA also enhances sperm kinematic parameters and increases hyper-activation, with similar magnitude of those produced by progesterone and mediated mainly by the GABAA receptor [12]. Thus GABA may be a physiological regulator of sperm function. GAT1 is also observed in testes, epididymis and sperms [13-18]. The function of GAT1 has been demonstrated, showing compromised reproductive capacity in the GAT1 transgenic mice. The explanation of compromised reproduction of the GAT1 transgenic mice may be due to disturbed maturation of sperms, compared with wild type mice [15]. Therefore it is necessary to determine the distribution and/or correlation of GABA and GAT1 in epididymis, which may shed light in the role of these molecules in the maturation of sperm, which could be useful for understanding of male infertility with potential application in the management of such patients.
Materials and Methods
Animals
Adult C57 mice (n=4) (aged 8 weeks) were kept at the standard condition, as described [19]. Temperature of 22±2 and diurnal cycle of 12 hours were maintained. All experimental protocols were approved by Animal Ethic Committee, Tongji Hospital, Tongji University School of Medicine.
Histology
Epididymis was collected following cervical dislocation sacrificing. The collected epididymis was fixed with 4% paraformaldehyde and embedded in paraffin. Paraffin-sections (7 μm) were cut using Leica microtome. The sections were blocked with the rabbit serum-free protein following dewaxing. GABA and GAT1 antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Sections were incubated with either primary anti-GABA or anti-GAT1 (1:50) overnight at 4. These sections were further incubated with the secondary m (biotinylated rabbit anti-goat IgG) at room temperature after three times PBS washing. The sections were incubated with the horseradish peroxidase-labeled streptavidin for 30 min (goat sreptavidin/peroxidase Kit-SP 9003, Zhongshan Goldenbridge Biotechnology, Co LTD, China). Detection was performed with the AEC substrate. The color development was stopped using washing the slides with running water. Finally, sections were counterstained with hematoxylin. The controls were performed by omitting the primary antibody. The slides were then photographed under Leica microscope. Semi-quantification of the immunohistochemistry was performed using Axioplan 2 image microscopic image analysis system (Zeiss Company, Germany).
Statistical analysis
SPSS13.0 was used for the statistical analyses. Data are presented as mean±SD. Comparisons were made using t test. p<0.05 was considered statistically significant.
Results
GABA and GAT1 immunohistochemistry in epididymis
There was overall GABA production in the whole epididymis, including caput, corpus, and cauda (Figure 1a). However, GABA production in caput (Figure 1b) was 1.7 or 1.6-fold stronger than that in corpus or cauda, respectively (Figures 1c and 1d). Interestingly there was no significant difference of GABA between corpus and cauda. Similarly, GAT1 was also distributed in the whole epididymis, with similar patterns to that of GABA (Figures 2a-2d). The control showed no special staining (Figures 3a-3d).
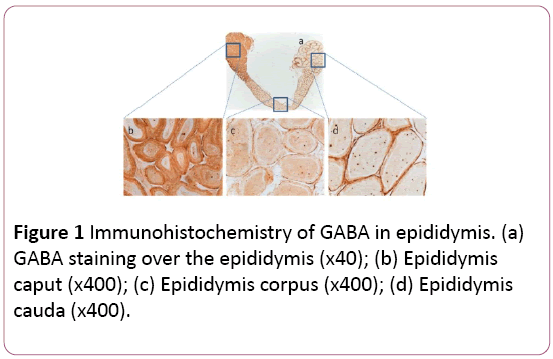
Figure 1: Immunohistochemistry of GABA in epididymis. (a) GABA staining over the epididymis (x40); (b) Epididymis caput (x400); (c) Epididymis corpus (x400); (d) Epididymis cauda (x400).
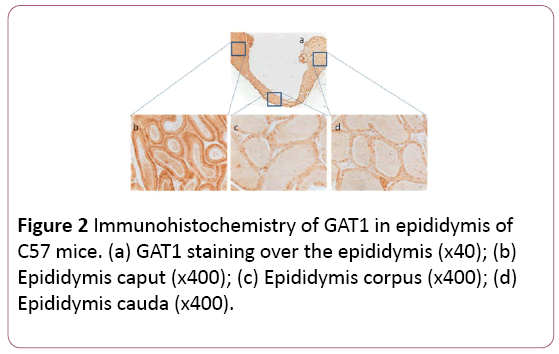
Figure 2: Immunohistochemistry of GAT1 in epididymis of C57 mice. (a) GAT1 staining over the epididymis (x40); (b) Epididymis caput (x400); (c) Epididymis corpus (x400); (d) Epididymis cauda (x400).
Figure 3: Control of immunohistochemistry in epididymis of C57 mice. (a) The whole epididymis (x40); Epididymis caput (x400); (c) Epididymis corpus (x400); (d) Epididymis cauda (x400).
Semiquantification of immunohistochemistry staining intensity of GABA and GAT1
The intensity of GABA staining varied from the caput 95.5 ± 6.5 to the corpus 55.9 ± 3.5 and the cauda 59.2 ± 4.2. The intensity of GAT1 varied from the caput 75.3 ± 2.0 to the corpus 52.0 ± 2.9 and the cauda 54.5 ± 1.7. The results identified that GABA distributed significantly (p<0.01) in the caput of epididymis, instead of the corpus and cauda. GAT1 has the similar distribution pattern as GABA (Figures 4a and 4b).
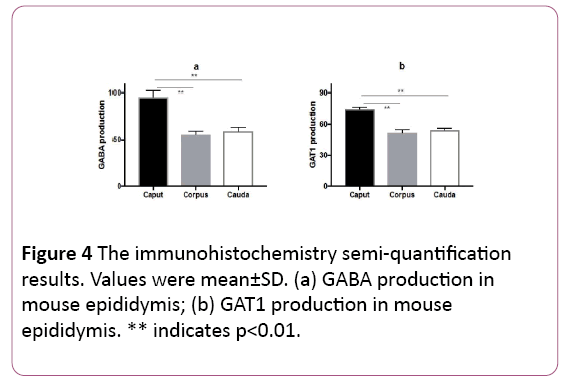
Figure 4: The immunohistochemistry semi-quantification results. Values were mean±SD. (a) GABA production in mouse epididymis; (b) GAT1 production in mouse epididymis. ** indicates p<0.01.
Discussion
GABA (expressed in sperms, testes and epididymis) plays an important role in spermiotelcosis [13-15]. Our previous studies demonstrate that there are abnormal histopathological presentations in testis and sperm from GAT1 transgenic mice. However, there are no remarkable changes in the epididymis [15]. This can explain that the male reproduction is compromised in GAT1 transgenic mice [15,16]. In the current study, we found that GABA and GAT1 distributed differentially in mouse epididymis caput, corpus, and cauda. Our current findings are in line with our previous two reports, showing that there was differential distribution of GABA, as well as, GAT1 in epididymis, particularly in caput, suggesting that GABA and GAT1 may contribute to the maturation of sperm and reproduction in male.
GAT1 is responsible for transferring GABA from the epididymis lumen into the epithelium cells for homeostasis in epididymis. Consistent distribution of GABA and GAT1 in epididymis caput, corpus, and cauda, might be due to that GAT1 regulates concentration of GABA in the epididymis [15].
The physiological function of epididymis is mainly for sperm maturation. Spermatozoa enter the epididymal tubule from the testis and, undergo a process of maturation that confers motility and the ability to fertilize ova during transit [18]. GABA is able to induce sperm acrosome reaction and increase sperm motility in vitro [7,10,12]. Acrosome reaction is a critical step for fertilization; whereas motility is also important for sperms to reach ova for fertilization. Thus the highest production of GABA/GAT1 in the epididymis caput is consistent with our previous published work, suggesting that GABA contributes to both acrosome reaction and sperm motility.
Conclusion
To conclude, our observation suggests that GABA and GAT1 play an important role in epididymal function. Such information is important for basic research in reproductive physiology, as well as, for clinical practice in Andrology.
21385
References
- Zorrilla J,Trigo FF, Kawaguchi SY (2017) Axonal GABAA receptors depolarize presynaptic terminals and facilitate transmitter release in cerebellar Purkinje cells. J Physiol.
- Damgaard M,Haugaard AS,Kickinger S, Al-Khawaja A, Lie MEK, et al. (2017) Development of non-GAT1-selective inhibitors: Challenges and achievements. Adv Neurobiol 16:315-332.
- Fuhrer TE,Palpagama TH,Waldvogel HJ, Synek BJL, Turner C,et al. (2017) Impaired expression of GABA transporters in the human Alzheimer's disease hippocampus, subiculum, entorhinal cortex and superior temporal gyrus.Neuroscience351:108-118.
- Biggs K,Seidel JS,Wilson A, Martyniuk CJ (2013) γ-Amino-butyricacid(GABA) receptor subunit and transporter expression in the gonad and liver of the fathead minnow (Pimephales promelas).Comp Biochem Physiol A Mol Integr Physiol166:119-127.
- Erdo SL, Nemet L, Szporny L (1983) The occurrence of GABA invas deferens, prostate, epididymis, seminal vesicle and testicle of therat. Acta Biol Hung 34:435.
- Shi QX, Yuan YY, Roldan ER (1997) γ-Aminobutyric acid (GABA) induces the acrosome reaction inhuman spermatozoa. Mol hum Reprod 3:677-683.
- Li S,Zhang Y,Liu H, Yan Y, Li Y (2008) Identificationand expression of GABACreceptorinrattestisandspermatozoa. Acta Biochim Biophys Sin40:761-767.
- Hu JH, Yan YC (2002) Identification of gamma1 subunit of GABA(A) receptor in rat testis. Cell Res 12: 33-37.
- Shi QX, Roldan ER (1995) Evidence that GABAA-like receptor is involved in progesterone-induced acrosome reaction exocytosis in mouse spermatozoa. Biol Reprod 52: 373-381.
- Calogero AE, Burrello N, Ferrara E (1999) Gamma-aminobutyric acid (GABA) A and B receptors mediated the stimulatory effects of GABA on the human sperm acrosome reaction: Interaction with progesterone. Fertil Steril 71: 930-936.
- Hu JH, He XB, Wu Q, Yan YC, Koide SS (2002) Biphasic effect of GABA on rat sperm acrosome reaction: involvement of GABA(A) and GABA(B) receptors. Arch Androl 48: 369-378.
- Calogero AE, Hall J, Fishel S, Green S, Hunter A, et al. (1996) Effects of gamma- aminobutyric acid on human sperm motility and hyperactivation. Mol Human Reprod 2: 733-738.
- Akinci MK, Schofield PR (1999) Widespread expression of GABAA receptor subunits in peripheral tissues. Neurosci Res 35: 145-153.
- Hu JH, He XB, Yan YC (2000) Identification of γ-aminobutyric acid transporter (GAT1) on the rat sperm. Cell Res 10: 51-58.
- Zhang JF, Gui YP, Yuan T, Bian C, Guo L, et al. (2009) Expression of GAT1 inmale reproductive system and its effects on reproduction in mice. Sys Biol in Reprod Med 55:175-180.
- Hu JH, Zhang JF, Ma YH, Jiang J, Yang N, et al. (2004) Impaired reproduction in transgenic mice overexpressing γ-aminobutyric acid transporter I (GAT1). Cell Res14: 54-59.
- Ma YH, Hu JH, Zhou XG, Mei ZT, Fei J, et al. (2000) γ-aminobutyric acid transporter (GAT1) overexpression in mouse affects the testicular morphology. Cell Res 10: 59-69.
- Hermo L, Robaire B (2002) Epididymal cell types and their function. In: RobaireB, Hinton BT (eds). The epididymis: From molecules to clinical practice. New York: Kluwer Academic/Plenum Press, USA. pp. 81–102.
- Liu HJ, Wise SG, Rnjak-Kovacina, Kaplan DL, Bilek MM, et al. (2014) Biocompatibility of silk-tropoelastin protein polymers. Biomaterials 35:5138-5147.










