Keywords
|
| Prenatal exposure; Late onset cancers; Complex magnetic field patterns; Adenocarcinoma; Fibrocarcinoma; Life-span |
Introduction
|
| Patterns of magnetic fields composed of aggregates of pulses whose durations are within the latency for base nucleotides to be added to DNA or RNA have the potential to influence gene expressions or their consequences. Karbowski et al., and Buckner [1,2] have shown that a frequency-modulated field known to produce analgesia in rats [3] or fields with similar spectral power density slow the growth of malignant cells but not that of normal cells in culture. Unpublished studies by members of our research group have shown that human breast cell lines MDA-231 and MCF- 7 show diminished growth in response to this specific pattern (FM) of magnetic fields. |
| Certain patterns of magnetic fields could be contributing factors for the induction of oncogenes Cfos ab1 and integrin alpha 5 beta 1 [4]. According to Wertheimer and Leeper [5] even 60 Hz magnetic fields when presented with specific wiring configurations increased the potential occurrence of childhood leukaemia. Power frequency (50 Hz, 60 Hz) fields at intensities encountered in many domiciles can enhance ornithine decarboxylase activity to levels induced by the carcinogen DMBA (dimethylbenz(a)anthracene) in the mammary gland [6]. Pulsed fields induce cellular transcription in dipteran salivary glands after 15 min of exposure [7] and modulate gene expression in myocardial cells [8]. These pulsed fields in general are more biologically effective than sine-waves [9-11], when presented at comparable intensities. |
| The rapidly dividing, migrating, and differentiating embryo-fetal system may be particularly sensitive to the effects of very weak but physiologically-patterned magnetic fields. St-Pierre and Persinger [12] constructed patterns of magnetic fields that were designed to converge with the intrinsic temporal complexity and fundamental energies of cell division and migration [13]. Micro-array analyses had indicated that the brains of mice exposed to a complexsequenced magnetic field for 3 wk displayed upregulation of prealbumin and mitogenic growth factor genes and down regulation of phosphotidylinositol 4-phosphate and ganglioside-induced differentiation-associated protein genes. They found that some but not all adult rats that had been exposed during their entire prenatal development to 30 to 50 nT magnetic fields designed to affect “gene expression” through this complex-sequenced pattern displayed conspicuous unusual cell arrangements within the hippocampal formation. The largest anomaly involved a heterotopia that occupied about one-fifth of one Ammon’s horn. The anomaly was composed of irregular shaped capillaries, oddly arranged neurons, and increased density of glia cells. St-Pierre and Persinger [12] suggested that the process could be related to the cleavage of brain specific extracellular matrix protein that could mediate or encourage the growth of gliomas [14]. |
| In the present study, we exposed rats during their entire prenatal development to one of two types of complex, weak magnetic fields whose structures were patterned after natural, physiologically relevant activity. The first was a frequency-modulated field that has been shown to induce analgesia in snails [15] and rats [3]. This same pattern reduced or eliminated the development of the growth of injected melanoma (B16-BL6) cells in mice [16]. The second pattern was custom-constructed, from both theory and a general appreciation of physiological patterns, to affect development. It was called the complex-sequenced field because it contained a series of different patterns each of which had been shown to be bioeffective. Rats that have been exposed to this pattern during their entire prenatal development exhibited conspicuous increases in open field activity [17] and much less proficient maze learning [18]. |
| To date few researchers have monitored rats that have been exposed to these potentially potent magnetic fields from birth until the geriatric period when spontaneous tumors become discernable. In the Wistar albino rat for example, we have observed for 30 years that female rats occasionally display adenocarcinomas or fibrocarcinomas, particularly in the vicinity of the mammary glands. In this study we monitored rats that had been exposed to either a single frequency-modulated field or to the complex-sequenced field throughout their life times until they were terminated upon reaching the “clinical” endpoint. Here we present evidence that the temporal pattern of the prenatally applied field can significantly alter the proportion of the population that displayed malignant growths (tumors) within the body. |
Materials and Methods
|
| A total of 165 rats (approximately equal numbers of males and females) were maintained in standard wire cages to monitor the emergence of conspicuous body tumors. Forty-five (45) of the rats had been exposed to the frequency-modulated field and 45 rats had been exposed to the complex-sequence field. These subjects had been randomly selected from 36 different litters. An additional 75 rats were selected from the sham field litters and from those born in the colony for other experiments. |
|
General procedure of prenatal exposure
|
| Over a one year period and in six separate blocks of experiments one male and one female were placed in each of four equally sized compartments. The characteristics of the cages and room have been described in detail elsewhere [12]. The male was removed after 8 days. At each end of the cage was a magnetic coil. The two coils (38 cm x 33 cm x 27 cm) were wrapped with 305 m of 30 AWG wire in a single layer (18 cm wide). The circuitry was designed so that only one coil would be activated for any given block of experiments. In the present experiments 3 of the experimental blocks involved activation of one coil while the other experimental blocks involved activation of the other coil. |
| During the subsequent 22 to 25 days until the litters had been born either the frequency-modulated pattern or the complex sequenced pattern was generated continuously [19]. The point duration for the frequency-modulation pattern was 3 ms. In cell culture this point duration produces the greatest inhibition of malignant cell growth. In other words, the duration each of the 849 points (voltages) that generated the field was 3 ms. For the complex-sequenced pattern the 9,999 points, each with 1 ms durations, were presented for 10 sec every 50 sec, i.e., 10 sec on, 40 sec off. The specific patterns of the magnetic fields were generated by converting columns of numbers, each number with a value between 0 and 255, into voltages by an XT personal computer. A value of 127 was 0 V while a value of 255 was +5 V and a value of 0 was -5 V. These numbers were then converted by a custom constructed device into voltages that were directed towards one of the two coils [20]. In other words, the physiologically-patterned magnetic fields were achieved by transforming the range of 256 numbers to appropriate voltages for discrete intervals from which the current was generated that activated the exposure coils. |
| The various visual patterns for the components of the complexsequenced have been reported previously [20]. The sequence was composed of 8 blocks. Within each of 6 blocks different patterns each lasting for 200 ms. The single “neutral” 200 ms segments were 0 V. Consequently, the total duration was 10 s. The names for the patterns in each block were: |
| 1. Burst LTP FM LTP Burst LTP FM LTP |
| 2. Burst LTP FM LTP Burst LTP FM LTP |
| 3. Neutral |
| 4. P-ring LTP N-ring LTP P-ring LTP N-ring LTP |
| 5. P-ring LTP N-ring LTP P-ring LTP N-ring LTP |
| 6. Neutral |
| 7. Fibonacci LTP 5 Hz LTP Fibonacci LTP 5 Hz LTP |
| 8. Fibonacci LTP 5 Hz LTP Fibonacci LTP 5 Hz LTP |
| The major component of the complex-sequenced field was the LTP pattern which was employed as a “spacer” and was composed of one pulse (primer) followed 173 msec later by 4 more pulses. This pattern was extracted exactly from the work of Rose, et al., [21] who found that electrical stimulation of hippocampal slices with this pattern produced reliable long-term potentiation (LTP). |
| The 1 msec point duration for the complex-sequenced field was based on the theoretical work of Fong, Wei, Persinger and Robb [22-24]. Fong considered each base of RNA to be an electric dipole. A pulse of 100 mV for 1 msec, which is long enough to act on the moving tape (RNA strand) for a distance of 0.5 nm (the width a base), would impart an energy of about 10-20 joules to a dipole. According to our approach the energy within the magnetic field is the operating vector where as the induced electric field (from Faraday effects) is less significant. For an averaged field strength of 300 nT (0.3 mG), for example, the energy per millisecond pulse within the volume of a cell with a width of 10 μm would be ~10-20 J per second. Or, if the 1 msec increment were critical, the required volume to generate such energy from this intensity would be within the equivalent width of 0.1 mm which would be sufficient to affect the early differentiating embryo and fetus. Theoretically a change or modification of only one triplet of base pairs could have permanent although recondite effects that might not be manifested until much later in the animal’s ontogeny. |
| The ranges of intensities of the complex magnetic fields within the four chambers decreased as a function of distance from the activated coil. The four successive ranges, from either activated coil, were 590 nT to 1150 nT (1.5 mG), 90 nT to 580 nT, 30 to 50 nT and 5 nT to 20 nT. These values, as measured by a Metex N380 meter coupled to a magnetic sensor did not differ appreciably between the two patterns. The presence of the fields was verified by direct measurement daily. In this paper these ranges defined the high intensity, medium intensity, low intensity, and very low intensity group, respectively. The rats from the 15 litters delivered in the frequency-modulated fields, the 18 litters delivered in the complex sequenced field, and the 4 litters exposed to the sham field were culled to 4 males and 4 females within 24 hr. At weaning (22 days) they were removed from their mothers and the experimental conditions and maintained in groups of two to three within same sex cages. Litters born in the colony that served as reference groups were also culled to 8 pups with more or less equal sexes in the same manner. |
| The rats were given water and Purina rat chow ad libitum for the remainder of their lives. When they reached the clinical endpoint, as discerned by the experimenters and the Animal Technicians as defined by sustained loss of weight, piloerection and lethargy for several days and other indicators familiar to experienced researchers the rat was terminated by decapitation, carbon dioxide, or barbiturate overdose depending upon the planned use of tissue. Tumors were verified and removed at the time of necropsy and further identified by routine histology. The tissue was fixed in ethanol-formalin-acetic acid, sectioned at 10 μm with a microtome and stained with Toluidine Blue O. Although all rats were allowed to live their entire lives for humanitarian reasons, the endpoint for collection of data was two years after the birth of the animal. The numbers of obvious overt tumours for the rats exposed to the frequency modulated field (3 ms point duration), complexsequence field (1 ms point duration) or ordinary colony and sham field (reference) conditions were assessed by loglinear analyses employing SPSS software. |
Results
|
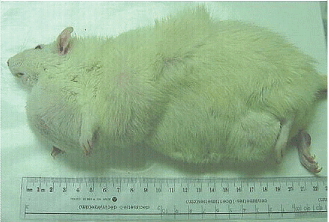
| A total of 17 male and female rats of the 90 rats (45 exposed to the frequency-modulated pattern and 45 exposed to the complex sequenced field) developed overt tumours some of which exceeded 100 g. Fifteen or 33% of the rats with tumours had been exposed to the complex-sequenced field. Eleven of these rats had been exposed to the low (30 to 50 nT; median = 40 nT) or medium (90 to 580 nT; median = 225 nT) intensities. In comparison two (female) or 4% of the rats that had been exposed to the frequency-modulated field during prenatal development displayed overt tumors. Only two (of 75) or 2.7% rat from the colony control and sham-exposed rats displayed any discernable overt tumor. Only those rats that had been exposed to the complex-sequenced field exhibited an unusual manifestation of multiple tumors that were evident over multiple sites on the body. An example is shown in Figure 1. Rats that had been exposed to the frequency-modulated pattern or colony controls never exhibited more than one tumor, if it emerged. |
| Hierarchical log-linear analysis as a function of type of pattern (complex-sequence vs frequency-modulated), intensity (very low, low, medium, and high) and presence of one discernable peripheral tumor (no, yes) demonstrated that the significant increase in proportion of tumors in rats that had been exposed to the complex-sequenced compared to the frequency-modulated field was significant statistically [chi-squared (1) =14.72, p <0.001]. There were no statistically significant disconcordances for the other interactions. The equivalent phi (correlation) coefficient was 0.37. |
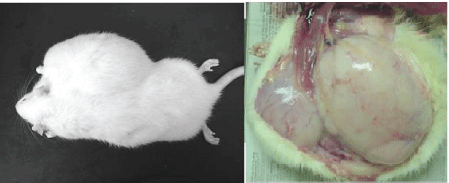
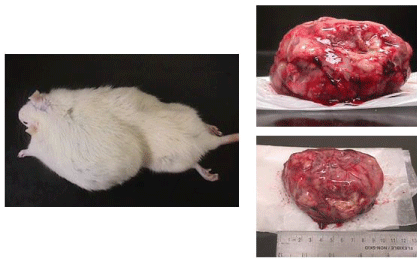
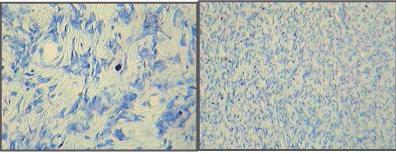
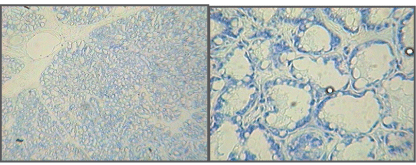
| Microscopic examination indicated cytology consistent with adenocarcinomas and fibrocarcinomas, the two most common tumours of the aging Wistar albino rat. Gross tissue examples of the two types are shown in Figures 2 and 3. Histological samples of each type of tumor are shown in Figures 4 and 5. |
Discussion
|
| To our knowledge this is the first major rodent study in which rats exposed during their entire prenatal development to a magnetic field pattern designed to affect energies related to junction of base nucleotides during DNA and RNA synthesis, which can involve energies as low as 0.1 eV or 1.6 × 10-20 J [25] were followed throughout their lifespan into the geriatric period. The complex-sequenced pattern resulted in a large proportion of the population displaying the class of mammary tumours that sometimes develops spontaneously in this population. However a second pattern (that has been shown to delay the growth of malignant cells in cultures and in mice) did not produce longterm changes in the proportion of tumor growth compared to colony reference groups. |
| The specificity of intensity (or more likely the induced energy associated with the magnetic field) within a narrow band was also indicated. Whereas the rats exposed prenatally to the sequenced-pattern magnetic fields within the 30 to 150 T intensity range developed more late onset tumors, those exposed to the same pattern but with either less (<30 nT) or greater (>580 nT) intensities did not display this increased prevalence. The prorated median range for the effective intensity band would have been 40 and 300 nT. Employing the classic energetic relation E=(B2/2 μ) × volume, were B is the magnetic field strength and μ is magnetic permeability, the energies within the volume of an average cell per pulse would be between 10-24 and 10-23 J. With approximately 1000 pulses of this energy per second (f) the integrated sum would be between 10-21 and 10-20 J per s, which is well within the potential range to interact the with the energies associated with adding base nucleotides. In other words the non-linearity effect of intensity could have reflected the discrete convergence with the “quantum” or increment of energy that would have most affected the molecular processes. Such non-linearity, as inferred by optimal Kd values where approximately 50% of the ligand is sequestered by a protein, defines the domain of receptor subtypes. |
| If the frequency component were not considered the energy would be between about 4 × 10-23 J to 6.3 × 10-25 J. The associated voltage (by dividing Joules by unit charge of 1.6 × 10-19 A·s) would be between 0.2 mV and 3 μV which is within the range associated with activation of single ion channels. On the other hand Faradic types of induction, frequently defined by V = B × f × area (m2) would indicate that for 1000 pulses per second and a typical cellular area (~8 × 10-11 m2) a range between 3 × 10-15 V to 2.4 × 10-14 V. The corresponding energy (by multiplying the unit charge) would be between 3.8 × 10-33 J to 5 × 10-34 J and would likely become significant as a “frequency” when divided by Planck’s constant (6.626 × 10-34 J·s). These “energetic frequencies” would be equivalent to periods between 170 ms and 1 s which was within the range of the individual increments (200 ms) that composed the complex-sequenced field. |
| Of course even though we designed the complex-sequenced field to converge with the intrinsic properties of dynamic cells, the similarities of quantified solutions do not prove the fields operated with this mechanism. The frequency modulated pattern was also associated with similar energies at the effective intensities. However there was no evidence of substantially increased proportions of tumor growth compared to “spontaneous” incidences within the colony population. This differential suggests that in addition to the energetic domains there are qualitative features, such as the temporal pattern, that must overlap with differentiating cells to be maximally disruptive. In addition to the complexity the major difference between the two patterns of fields was their duration before repetition. The complex pattern that increased the incidence of geriatric tumors was repeated every 10s compared to the simpler pattern that was repeated ever approximately 2.5s. That wave parameters can differentially affect ion/ligand binding kinetics and intermittent noise generated within the applied field can alter enzymatic activity has been well established [9]. |
Acknowledgement
|
| Thanks to Professors Lukasz Karbowski, Niosha Murugan and Christopher Blomme and to Dr. M. J. Dupont for technical contributions. |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |
| |










