Short Communication - (2022) Volume 13, Issue 4
Does the unified Parkinson's disease rating scale adequately estimate severity of dysarthria?
Kristie A Spencer1*,
Katherine A Brown2 and
Gillian Elder1
1Department of Speech & Hearing Sciences, University of Washington, Seattle, WA 98105, USA
2Department of Communication Sciences and Disorders, Augustana College, Rock Island, IL 61201, USA
*Correspondence:
Kristie A Spencer, Department of Speech & Hearing Sciences, University of Washington, Seattle, WA 98105,
USA,
Email:
Received: 25-Mar-2022, Manuscript No. ipjnn-22-12688;
Editor assigned: 27-Mar-2022, Pre QC No. P-12688;
Reviewed: 19-Apr-2022, QC No. Q-12688;
Revised: 23-Apr-2022, Manuscript No. R-12688;
Published:
30-Apr-2022
Abstract
Background: Speech decline is a common and detrimental
complication of Parkinson’s disease (PD). The Unified Parkinson’s
Disease Rating Scale (UPDRS) is typically used by the medical
community to gauge the presence and severity of PD symptoms,
including dysarthria. Accurately tracking the presence and severity
of dysarthria has important implications for differential diagnosis,
disease course, and therapeutic response.
Objectives: To determine the relationship between Movement
Disorder Society (MDS) UPDRS ratings and gold standard speech
intelligibility transcription scores.
Methods: Twenty-seven speakers with PD provided monologue
speech samples. MDS-UPDRS ratings of speech were compared to
average speech intelligibility scores attained by three naïve judges.
Results: MDS-UPDRS ratings and speech intelligibility calculations
were significantly correlated.
Conclusion: The significant relationship between these two
severity indicators provides preliminary evidence of criterion validity
and suggests that the single MDS-UPDRS question is reflective of
overall speech severity as determined by the gold standard of mean
intelligibility transcription scores.
Keywords
Parkinson; Dysarthria; Speech; Intelligibility; UPDRS
Introduction
Parkinson’s disease (PD) is a progressive neurodegenerative
disorder characterized by a heterogeneous spectrum
of motor and non-motor characteristics [1]. Accurate
measurement of the symptoms of PD is important for
tracking disease progression and therapeutic response and
is most often conducted using the Unified Parkinson’s
Disease Rating Scale (UPDRS). This scale, first published
in 1987, was revised in 2008 by the International Parkinson
and Movement Disorder Society (MDS) [2,3]. At present,
the MDS-UPDRS is the most widely used scale across
clinical and research settings [4,5].
In both versions of the UPDRS scale, speech decline
is captured with a single question. Speech disruption, or
dysarthria, is highly prevalent in PD [6]. It leads to reduced
speech intelligibility and has negative consequences for
overall well-being and involvement in daily life, including
social isolation [7-9]. The speech changes experienced
by individuals with PD are variable in their nature and
severity, and can differentially impact a wide array of
subsystems, including respiration, phonation, articulation,
and prosody. As such, detrimental and complex changes can
be manifested in rate of speech, precision of articulation,
voice quality, fluency, pitch variability, loudness level, and
so forth [10].
There are few and conflicting reports about the
ability of the UPDRS to accurately reflect the presence
and severity of dysarthria [11-13]. In the dysarthria
literature, speech intelligibility, or how understandable a
speaker is to a listener, is often used as a proxy for speech
severity [14]. To determine intelligibility levels, speaking
passages are transcribed and the percent of understood
words is determined by a naïve listener(s). Particularly in
research settings, intelligibility is often calculated across
speaking tasks as elicitation method is known to influence
understandability in speakers with PD [15,16]. In contrast,
the MDS-UPDRS question captures speech severity using
a 5-point scale, representing no speech change or slight,
mild, moderate, severe difficulty understanding speech [3].
It is unknown whether the UPDRS speech scale will
parallel the more established, gold standard metric of
intelligibility in its ability to represent speech decline in PD
[17]. Thus, this study investigated the relationship between
MDS-UPDRS speech ratings and speech intelligibility
calculations. Given the negative impact of dysarthria
to quality of life, it is important to understand whether
a global speech rating is sufficiently sensitive to speech
decline [7-9].
Additionally, early presence of dysarthria is more
suggestive of atypical parkinsonian disorders underscoring
the importance of accurate identification of dysarthria to the
differential diagnosis process [18,19]. Finally, the potential
of dysarthria and other axial motor symptoms to inform
disease course, such as more rapid progression to dementia,
has been reported, elevating the need to accurately capture
this secondary motor symptom of PD [20-22].
Methods
Participants
Participants were 27 speakers with PD with an average
age of 71.11 years and average disease duration of 9.06
years (Tab. 1). Inclusion criteria were an established
diagnosis of PD by a neurologist with no other neurological
complications (e.g., stroke, traumatic brain injury),
presence of dysarthria without dyskinesias that would affect
speech performance, minimum age of 50 years, and the
ability to pass a vision, hearing, and depression screening.
Participants were excluded for atypical Parkinsonism
and young onset PD. All participants were in the ON
medication state during examinations. Detailed participant
characteristics are reported elsewhere [23].
| Participant |
Age |
Sex |
Years of
Education |
Disease
Duration
(Yrs) |
Speech
Intelligibility
(100) |
MDSUPDRS
Rating |
| 01 |
77 |
M |
20 |
12 |
87.67 |
3 |
| 02 |
80 |
M |
18 |
8 |
89.00 |
1 |
| 03 |
68 |
F |
17 |
16 |
88.00 |
1 |
| 04 |
69 |
M |
19 |
8 |
87.00 |
2 |
| 05 |
70 |
M |
16 |
8 |
81.33 |
3 |
| 06 |
76 |
M |
16 |
18 |
95.67 |
1 |
| 07 |
74 |
M |
16 |
15 |
93.00 |
2 |
| 08 |
66 |
F |
17 |
9 |
88.33 |
2 |
| 09 |
73 |
M |
16 |
8 |
94.00 |
1 |
| 10 |
67 |
M |
16 |
6.5 |
96.00 |
1 |
| 11 |
63 |
M |
16 |
4 |
100 |
1 |
| 12 |
66 |
M |
18 |
17 |
99.00 |
1 |
| 13 |
70 |
M |
16 |
13 |
97.33 |
0 |
| 14 |
73 |
M |
15 |
2 |
94.67 |
1 |
| 15 |
69 |
F |
17 |
1 |
98.67 |
1 |
| 16 |
80 |
M |
16 |
11 |
97.33 |
1 |
| 17 |
76 |
F |
18 |
4 |
95.00 |
0 |
| 18 |
71 |
F |
21 |
7 |
96.67 |
1 |
| 19 |
65 |
F |
22 |
8 |
97.67 |
1 |
| 20 |
81 |
F |
18 |
20 |
96.67 |
1 |
| 21 |
62 |
M |
18 |
7 |
99.00 |
1 |
| 22 |
64 |
M |
14 |
4 |
85.33 |
1 |
| 23 |
60 |
M |
16 |
4 |
89.00 |
0 |
| 24 |
73 |
F |
16 |
6 |
95.67 |
1 |
| 25 |
72 |
M |
18 |
10 |
93.67 |
1 |
| 26 |
75 |
M |
13 |
11 |
94.33 |
2 |
| 27 |
80 |
F |
18 |
10 |
97.00 |
2 |
Mean
(SD) |
71.11
(5.78) |
M=18
F=9 |
17.07
(1.92) |
9.06
(4.82) |
93.82
(4.87) |
1.25
(2.75) |
Tab. 1. Demographic and speech severity ratings across participants.
Procedure
Intelligibility calculations: A monologue was elicited by asking participants to talk about their job, their family or a
vacation for approximately 60 seconds. Use of a monologue
is considered best practice for its ecological validity and is
recommended for speakers with PD and mild-moderate
speech decline [16]. Samples were segmented into speech
runs, which are operationally defined as a stretch of speech
bounded by a silent period or pause between words of
at least 200 milliseconds [24]. Each monologue was
transcribed to identify the first 100-word speech run that
did not contain proper nouns, formulaic phrases or specialty
vocabulary [25]. An independent judge reassessed 15% of
the speech run coding; interjudge reliability was 97.8%.
Speech was recorded using a high-quality, head-mounted
microphone (AKG C520) with a constant mouth-tomicrophone
distance of two inches [23]. The microphone
was connected to a portable digital speech recorder (Zoom
H6, GU- ZOOMH6). All speech samples were recorded
in a quiet environment with low ambient noise.
The transcribed, 100-word samples were then used
for intelligibility scores. Transcriptions of the dysarthric
speech were conducted by three naïve listeners to provide
a mean intelligibility rating for each speaker with PD.
Listeners were native English speakers without hearing loss.
Intelligibility scores were determined by counting
the number of correctly identified words and dividing
by the total of 100 words, using established transcription procedures [26]. Misspellings and homonyms were
considered correct; synonyms or morphological variations
were considered incorrect [26].
MDS-UPDRS calculations: UPDRS ratings were
completed independently using the monologue speech
samples by a MDS-UPDRS trained speech expert (first
author) who was blinded to intelligibility scores.
Statistical analysis: Pearson correlation (bivariate
analysis) was used to determine the relationship between
MDS-UPDRS ratings and Intelligibility scores.
Results
As can be seen in Tab. 1, the mean of the intelligibility
scores from the monologues was 93.82 (SD= 4.87) with
scores ranging from 81.33 – 100%. MDS-UPDRS ratings,
based on the monologues, averaged 1.25 (SD=2.75)
and ranged from 0-3. Collectively, these indices suggest
that the speakers with PD had mild to moderate speech
impairment.
MDS-UPDRS ratings and speech intelligibility
calculations were found to have a significant moderate
negative correlation (r(25) = - 0.48, p =0.012). As illustrated
in the scatter plot (Fig. 1), higher intelligibility scores were
associated with lower (better) MDS-UPDRS ratings.
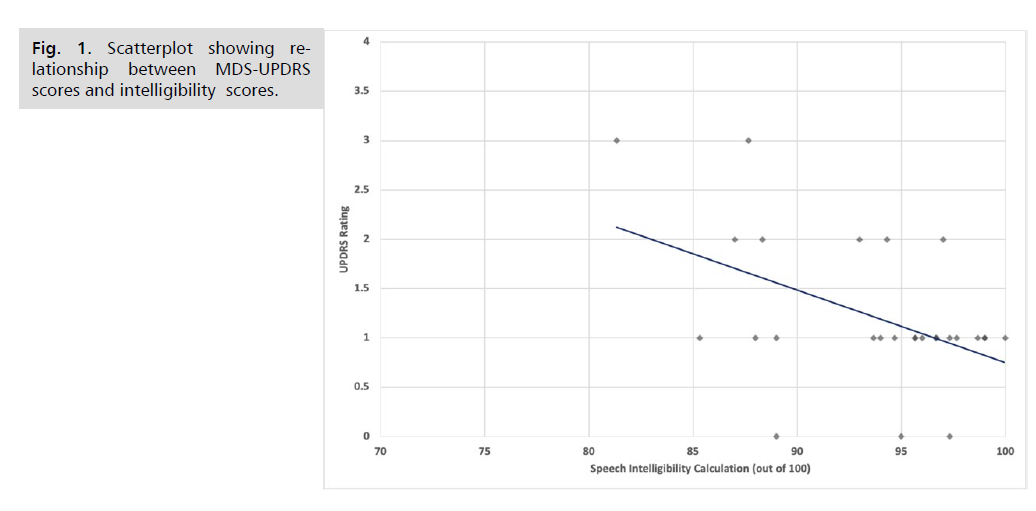
Fig 1: Scatterplot showing relationship between MDS-UPDRS scores and intelligibility scores.
Discussion
Neurologists and other medical professionals frequently
use the comprehensive MDS-UPDRS to characterize and
track severity and areas of decline in PD. As the domain
of speech is represented with a single question within
this measurement tool, it was uncertain whether UPDRS
ratings would correspond with the more established severity
metric of speech intelligibility. The significant relationship
between these two severity indicators provides preliminary
evidence of criterion validity and suggests that the MDSUPDRS
5-point scale is reflective of overall speech severity
as determined by the gold standard of mean intelligibility
scores. These findings align with current research suggesting that speech pathologist ratings of speech severity (normal,
mild, moderate, severe, profound) were strongly associated
with severity-surrogate measures of speech intelligibility,
speaking rate, and listener effort [13]. Thus, clinicians
can have increased confidence that the presence and
progression of speech decline is sufficiently captured by the
MDS-UPDRS speech scale.
Limitations
The current study is limited by a relatively small sample
as well as restricted speech and disease severity. Studying
a larger population of more severely impaired speakers
would provide insight into whether speech intelligibility
scores are influenced by the severity of PD and dysarthria.
Additionally, it is currently unknown whether UPDRS
speech ratings and speech intelligibility scores are equally
sensitive to change with treatment. Finally, it is important
to understand how the UPDRS speech scale compares to
the validity and reliability of similar rating scales, such as
the Communication Effectiveness Survey [27].
Acknowledgments
This work was supported by the Washington
State Parkinson Disease Registry and the Pat Tillman
Foundation (author KB). The authors would like to thank
Clare Friedlander for her contributions to the original
study.
Author Roles
1. Author 1 (Spencer): Research project conception,
design; Review and critique of statistical analysis, writing
draft of manuscript.
2. Author 2 (Brown): Conception, design and
execution of research project; Design and execution of
statistical analysis; Review and critique of draft.
3. Author 3 (Elder): Execution of research project;
Review and critique of statistical analysis and manuscript.
Disclosures
Funding sources and conflicts of interest:
No specific funding was received for this work. The
authors declare that there are no conflicts of interest
relevant to this work.
Financial disclosures for the previous 12 months:
Spencer: Received salary from the University of
Washington and royalties from Medical Speech Language
Pathology Book Series through Plural Publishing.
Brown: Received stipend from the University of
Washington and salary from Augustana College.
Elder: Graduate student; no additional disclosures to
report.
Ethical Compliance Statement
The University of Washington Institutional Review
Board approved this study. Written consent was secured
for all participants.
REFERENCES
- Thenganatt MA, Jankovic J. Parkinson Disease Subtypes. JAMA Neurol. 2014;71(4):499-504.
Google Scholar, Crossref, Indexed at
- Fahn S, Elton R, MotUD C. Unified Parkinson’s disease rating scale. In Fahn S, Marsden C, Calne D, Goldstein M, eds. Recent Development in Parkinson’s Disease. Florhan Park NJ, Macmillan Health Care Information. 1987;pp:153-164.
- Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23(15):2129-2170.
Google Scholar, Crossref
- Tosin MHS, Stebbins GT, Comella C, et al. Does MDS-UPDRS provide greater sensitivity to mild disease than UPDRS in De Novo Parkinson’s disease? Mov Disord Clin Pract. 2021;8(7):1092-1099.
Google Scholar, Crossref, Indexed at
- Rajan R, Brennan L, Bloem BR, et al. Integrated care in Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2020;35(9):1509-1531.
Google Scholar, Crossref, Indexed at
- Sapir S. Multiple factors are involved in the dysarthria associated with Parkinson's disease: a review with implications for clinical practice and research. J Speech Lang Hear Res. 2014;57(4):1330-1343.
Google Scholar, Crossref, Indexed at
- Spencer KA, Friedlander C, Brown KA. Predictors of health-related quality of life and communicative participation in individuals with dysarthria from parkinson's disease. Int J Neurodegener Dis. 2020;3(1).
Google Scholar, Crossref, Indexed at
- Mach H, Baylor C, Pompon RH, et al. Third-party disability in family members of people with communication disorders associated with Parkinson's disease. Topics Lang Dis. 2019;39(1):71-88.
Google Scholar
- Karlsen KH, Tandberg E, Arsland D, et al. Health related quality of life in Parkinson's disease: a prospective longitudinal study. J Neurol Neurosurg Psychiatry. 2000;69:584-589.
Google Scholar, Crossref, Indexed at
- Tjaden K. Speech and swallowing in Parkinson's disease. Topics Ger Rehab. 2008;24(2):115-126.
Google Scholar, Crossref, Indexed at
- Martinez-Martin P, Gil-Nagel A., Gracia LM et al. Unified Parkinson’s Disease Rating Scale characteristics and structure. Mov Disord. 1994;9(1):76-83.
Google Scholar, Crossref, Indexed at
- Richards M, Marder K, Cote L, et al. Inter-rater reliability of the Unified Parkinson’s Disease Rating Scale motor examination. Mov Disord. 1994;9(1):89-91.
Google Scholar, Crossref, Indexed at
- Zraick RI, Dennie TM, Tabbal SD, et al. Reliability of speech intelligibility ratings using the Unified Parkinson Disease Rating Scale. J Med Speech Lang Pathol. 2003;11(4):227-240.
Google Scholar, Indexed at
- Stipancic KL, Palmer KN, Rowe HP, et al. “You say severe, I say mild”: Toward an empirical classification of dysarthria severity. J Speech Lang Hear Res. 2021;64: 4718-4735.
Google Scholar, Crossref
- Sidtis DVL, Cameron K, Sidtis JJ. Dramatic effects of speech task on motor and linguistic planning in severely dysfluent parkinsonian speech. Clin Ling Phon. 2012;26(8):695-711.
Google Scholar, Crossref, Indexed at
- Weir-Mayta P, Spencer KA, Eadie TL, et al. Internally versus externally cued speech in Parkinson's disease and cerebellar disease. Am J Speech Lang Pathol. 2017;26(2S):583-595.
Google Scholar, Crossref, Indexed at
- Sussman JE, Tjaden K. Perceptual measures of speech from individuals with Parkinson’s disease and multiple sclerosis: Intelligibility and beyond. J Speech Lang Hear Res. 2012;55(4):1208-1219.
Google Scholar, Crossref, Indexed at
- Kowalska-Taczanowska R, Friedman A, Koziorowski D. Parkinson's disease or atypical parkinsonism? The importance of acoustic voice analysis in differential diagnosis of speech disorders. Brain Behav. 2020;10(8):e01700.
Google Scholar, Crossref, Indexed at
- Müller J, Wenning GK, Verny M, et al. Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Arch Neurol. 2001;58(2):259-264.
Google Scholar, Crossref, Indexed at
- Bugalho P, Viana-Baptista M. Predictors of cognitive decline in the early stages of Parkinson’s disease: A brief cognitive assessment longitudinal study. Parkin Dis. 2013;2019: 912037.
Google Scholar, Crossref, Indexed at
- Elgh E, Domellöf M, Linder J, et al. Cognitive function in early Parkinson’s disease: A population-based study: Cognition in Parkinson. Euro J Neurol. 2009;16(12):1278-1284.
Google Scholar, Crossref, Indexed at
- Schneider JS, Sendek S, Yang C. Relationship between motor symptoms, cognition, and demographic characteristics in treated mild/moderate Parkinson’s disease. PLos One. 2015;10(4):e0123231.
Google Scholar, Crossref, Indexed at
- Brown KA, Spencer KA. The relationship between speech characteristics and motor subtypes of Parkinson’s disease. Am J Speech Lang Pathol. 2020;29(4):2145-2154.
Google Scholar, Crossref, Indexed at
- Tjaden K, Wilding G. Effects of Speaking Task on Intelligibility in Parkinson's Disease. Clin Linguist Phon. 2011;25(2):155-168.
Google Scholar, Crossref, Indexed at
- Sidtis D, Cameron K, Bonura L, et al. Speech intelligibility by listening in Parkinson speech with and without deep brain stimulation: Task effects. J Neuroling. 2011;25(2):121-132.
Google Scholar, Crossref, Indexed at
- Bunton K, Keintz CK. The use of a dual-task paradigm for assessing speech intelligibility in clients with parkinson disease. J Med Speech Lang Pathol. 2008;16(3):141-155.
Google Scholar, Indexed at
- Donovan NJ, Velozo C, Rosenbek JC. The communicative effectiveness survey: Investigating its item-level psychometric properties. J Med Speech Lang Pathol. 2007;15(4):433-447.
Google Scholar, Indexed at






