George Umemoto1*, Hirokazu Furuya2, Yoshio Tsuboi3, Shinsuke Fujioka3, Hajime Arahata4, Miwa Sugahara4, Akihiro Watanabe4 and Mitsuaki Sakai4
1Department of Oral and Maxillofacial Surgery, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
2Department of Neurology, Faculty of Medicine, Kochi University Medical School, Kochi, Japan
3Department of Neurology, Faculty of Medicine, Fukuoka University, Fukuoka, Japan
4Department of Neurology, Neuro-Muscular Center, National Omuta Hospital, Fukuoka, Japan
*Corresponding Author:
George Umemoto
Department of Oral and Maxillofacial Surgery Faculty of Medicine, Fukuoka University, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
Tel: +81-92-801-1011
Fax: +81-92-801-1044
E-mail: george@minf.med.fukuoka-u.ac.jp
Received date: December 11, 2016; Accepted date: January 09, 2017; Published date: January 13, 2017
Citation: Umemoto G, Furuya H, Tsuboi Y, et al. Dysphagia in Multiple System Atrophy of Cerebellar and Parkinsonian Types. J Neurol Neurosci. 2017, 8:1. doi: 10.21767/2171-6625.1000165
Keywords
Multiple system atrophy; Cerebellar type; Parkinsonian type; Activities of daily living; Functional dysphagia scale; Tongue pressure
Introduction
Dysphagia, which is the most frequent complication of multiple system atrophy (MSA), is observed a short time after disease onset [1]. Evaluations and the appropriate treatment of patients suffering from dysphagia may prevent or at least delay other complications, such as aspiration pneumonia, and thereby improve their quality of life and increase survival time. A study by Higo et al. implied that the swallowing function in MSA patients may be more influenced by Parkinsonism than by cerebellar dysfunction and that Parkinsonism mainly affects the oral phase of swallowing [2]. They suggested that dysphagia in patients with MSA with predominant Parkinsonism (MSA-P) is caused by Parkinsonism, whereas in patients with MSA with predominant cerebellar ataxia (MSAC), the progression of the cerebellar dysfunction and overlapping Parkinsonism worsens the tongue movements. However, there are few studies that have evaluated dysphagia in patients with MSA and compared patients with the two types, MSA-C and MSA-P.
A previous study has shown that the time from the initial symptom to the appearance of other symptoms, indicating the evolution to MSA, is strongly related to the deterioration of activities of daily living (ADL) and the worsening of survival [3]. However, the relationship between swallowing dysfunction and ADL deterioration is not yet clear. Clarifying the correlation between dysphagia and ADL, and the potential differences between patients with MSA-C and MSA-P could provide valuable clues for effective treatments. Some previous studies tried to clarify the difference in various characteristics between MSA-C and MSA-P [4,5], but there were few studies to make comparison of swallowing function between the two subtypes. This study aimed to demonstrate the relationship between the degree of dysphagia and deterioration in ADL in patients with MSA by comparing patients with MSA-C and MSA-P.
Patients and Methods
Participants
We studied 61 patients with MSA (25 males and 36 females; mean ± standard deviation (SD) age=66.3 ± 11.0 years; range, 38−91 years) who were recruited from the Department of Neurology of the Faculty of Medicine of Fukuoka University and the Department of Neurology of the Neuro-Muscular Center of National Omuta Hospital. Neurologists from these hospitals determined the clinical diagnosis of probable or definite MSA based on the Consensus Conference statement concerning MSA diagnostic criteria [6]. Furthermore, the patients consisted of 40 with MSA-C (17 males and 23 females; mean ± SD age=65.4 ± 10.6 years; range, 38−85 years) and 21 with MSA-P (8 males and 13 females; mean ± SD age=68.1 ± 11.7 years; range, 45−91 years). Patients with other neurologic disorders, neuropsychological dysfunctions, and/or head and neck cancers, and those undergoing tube feeding were excluded. We checked the disease duration and assessed the activities of daily living of each patient on the Japanese version of the modified Barthel Index [7]. The modified Barthel Index is commonly used to assess patient’s capacity to perform 10 daily tasks without assistance and the patients were scored with a sum of 100. The Japanese version of the modified Barthel Index demonstrated satisfactory factorial validity and internal consistency in elderly people living at home [7]. The ethics committees of Fukuoka University Hospital and National Omuta Hospital approved the study, and an informed consent was obtained from all participants.
Measurement of tongue pressure
A handy probe was used in the tongue pressure measurement system (JMS Co. Ltd, Hiroshima, Japan) [8]. The probe was assembled with a small balloon and pressurized with air at 19.6 kPa. Participants were asked to compress the balloon onto their palate for approximately 7 s using the maximum voluntary effort of their tongue. The increase in the inner pressure of the balloon was measured as the tongue pressure. These tests were conducted thrice in a row and mean values were obtained.
VF examination
The swallowing function was evaluated by a modified barium swallow procedure and videofluoroscopy (VF) imaging [9]. It was recorded through lateral projection onto a DVD recorder (DIGA; Panasonic Corporation, Osaka, Japan) running at 30 frames/s. Each participant swallowed 5 mL each of barium water and barium gelatinous jelly while in the lateral position and was required to repeatedly swallow until all bolus pass through the pharynx. The recordings of swallowing barium water and barium gelatinous jelly were analyzed frame-by-frame and scored on the basis of the VF dysphagia scale with a sum of 100 (Table 1) [10]. We measured the time from start of masticating gelatinous jelly until passage of the jelly tip through the esophageal entrance as oropharyngeal transit time and studied the tongue root and mandible movements during the oropharyngeal transit time.
The measurements of bolus transit and the movements of the tongue root and mandible were completed by analyzing the recordings of the swallow trials frame-by-frame or in slow motion using movement analysis software in two dimensions (Dipp-Motion Pro, Ditect Corporation, Tokyo, Japan).
To evaluate mandibular movement, the movement of the most inferior point of the mandible was measured on the Yaxis, which was based on two optional points on the cervical vertebrae (Figure 1). To evaluate tongue root movement, the movement of the point where the tongue line and the mandible line intersected was calculated on the X-axis, which was vertically directed toward the Y-axis. The values of the evaluation were divided by the values of the oropharyngeal transit time, which was taken as a measure of the speed of the tongue root and mandibular movements [11].
| Parameter |
Coded value |
Score |
| Oral phase score |
| Lip closure |
Intact |
0 |
4 |
| Inadequate |
2 |
|
| None |
4 |
|
| Bolus formation |
Intact |
0 |
6 |
| Inadequate |
3 |
|
| None |
6 |
|
| Mastication |
Intact |
0 |
8 |
| Inadequate |
4 |
|
| None |
8 |
|
| Apraxia |
None |
0 |
4.5 |
| Mild |
1.5 |
|
| Moderate |
3 |
|
| Severe |
4.5 |
|
| Tongue-to-palate contact |
Intact |
0 |
10 |
| Inadequate |
5 |
|
| None |
10 |
|
| Premature bolus loss |
None |
0 |
4.5 |
| <10% |
1.5 |
|
| 10% to 50% |
3 |
|
| >50% |
4.5 |
|
| Oral transit time |
<1.5 |
0 |
3 |
| >1.5 |
3 |
|
| Pharyngeal phase score |
| Triggering of pharyngeal swallow |
Normal |
0 |
4.5 |
| Delayed |
4.5 |
|
| Vallecular residue |
None |
0 |
6 |
| <10% |
2 |
|
| 10% to 50% |
4 |
|
| >50% |
6 |
|
| Laryngeal elevation |
Normal |
0 |
9 |
| Impaired |
9 |
|
| Pyriform sinus residue |
None |
0 |
13.5 |
| <10% |
4.5 |
|
| 10% to 50% |
9 |
|
| >50% |
13.5 |
|
| Coating of pharyngeal wall |
No |
0 |
9 |
| |
Yes |
9 |
|
| Pharyngeal transit time |
<1.0s |
0 |
6 |
| >1.0s |
6 |
|
| Aspiration |
None |
0 |
12 |
| Supraglottic penetration |
6 |
|
| Subglottic aspiration |
12 |
|
| Total |
|
|
100 |
Table 1 Video-fluoroscopic dysphagia scale.
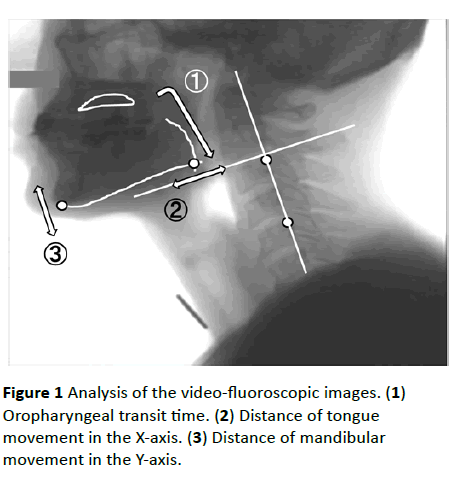
Figure 1 Analysis of the video-fluoroscopic images. (1) Oropharyngeal transit time. (2) Distance of tongue movement in the X-axis. (3) Distance of mandibular movement in the Y-axis.
Data analysis
The Mann–Whitney U tests were used to compare the data sets of the two groups.
Spearman rank correlation coefficients were used to measure the linear relationships between the ADL score and the other data, between the disease duration and the other data, and between the VF findings and tongue pressure. One patient with MSA-C could not complete the tongue pressure examination and the correlation coefficients were calculated excluding the missing value.
Statistical data were analyzed with the Statistical Package for the Social Sciences (SPSS 13.0 J) for Windows (IBM Corporation, Armonk, NY, USA), and p values less than 0.05 were considered significant.
Results
No significant differences between the MSA-C and MSA-P patients in age (65.4 ± 10.6 years vs. 68.1 ± 11.7 years; p=0.403), disease duration (4.05 ± 4.19 years vs. 3.50 ± 3.36 years; p=0.420), ADL score (53.8 ± 32.4 vs. 50.2 ± 30.8; p=0.465), dysphagia score (13.9 ± 12.3 vs. 16.3 ± 12.8; p=0.362), or tongue pressure (24.0 ± 10.4 kPa vs. 18.0 ± 11.5 kPa; p=0.064) were observed. The mean tongue pressure values of these MSA patients were lower than healthy people’s values (41.9 ± 9.9 kPa in the thirties, 40.4 ± 9.8 kPa in the forties, 40.7 ± 9.8 kPa in the fifties, 37.6 ± 8.8 kPa in the sixties, and 31.9 ± 8.9 kPa in the seventies) [12]. Although there was also no significant difference in the range of the tongue root (15.2 ± 7.0 mm/s vs. 15.5 ± 10.3 mm/s; p=0.430) and mandibular movements (21.1 ± 11.3 mm/s vs. 22.3 ± 16.2 mm/s; p=0.891), the patients with MSA-P showed a significantly longer oropharyngeal transit time than the patients with MSA-C (Figure 2; MSA-P, 10.2 ± 7.5 s vs. MSA-C, 6.7 ± 4.7 s; p=0.049).
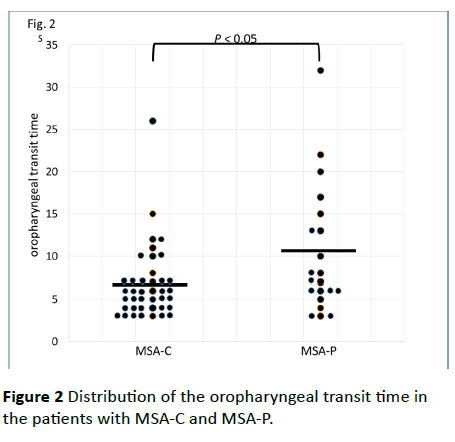
Figure 2 Distribution of the oropharyngeal transit time in the patients with MSA-C and MSA-P.
Significant correlations were found between the dysphagia score and ADL score (Figure 3; R=−0.654, p<0.001), between the ADL score and the tongue pressure (Figure 4; R=0.600, p<0.001), and between the dysphagia scores and the tongue pressure (Figure 5; R=−0.609, p<0.001), in the 61 patients. There was a significant correlation between the ADL score and the disease duration (R=−0.300, p=0.020). The disease duration did not correlate with the tongue pressure (R=0.200, p=0.125) but with the dysphagia score (R=0.258, p=0.046), especially with the pharyngeal phase score of MSA-P (Figure 6; R=0.613, p=0.006). Furthermore, the oropharyngeal transit time and the tongue pressure in the MSA-C group (Figure 7; R= −0.457, p=0.005) was significantly correlated. On the other hand, significant correlations between the tongue root movement and the tongue pressure (Figure 8; R=0.458, p=0.040), and between the mandibular movement and the tongue pressure (Figure 8; R=0.538, p=0.016) were observed in patients with MSA-P.
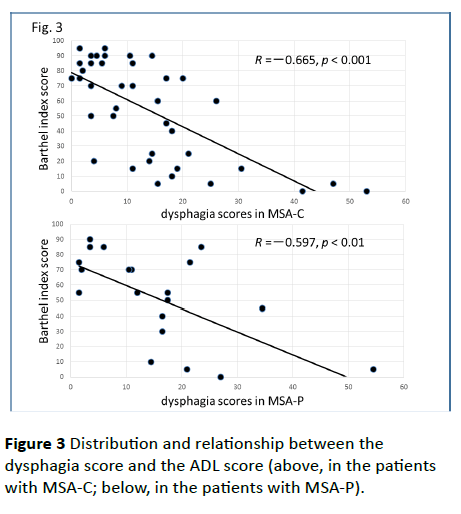
Figure 3 Distribution and relationship between the dysphagia score and the ADL score (above, in the patients with MSA-C; below, in the patients with MSA-P).
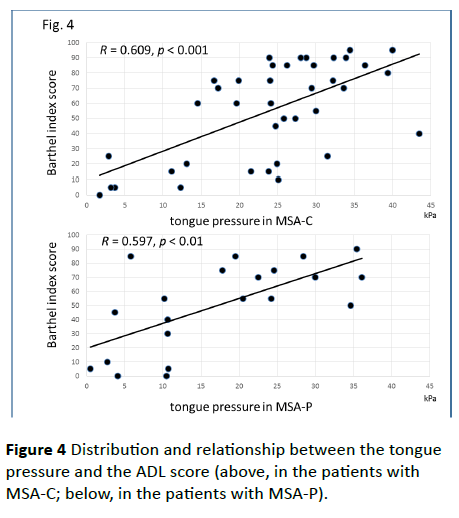
Figure 4 Distribution and relationship between the tongue pressure and the ADL score (above, in the patients with MSA-C; below, in the patients with MSA-P).
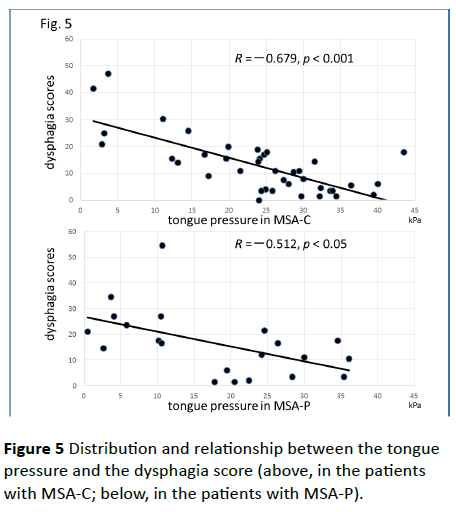
Figure 5 Distribution and relationship between the tongue pressure and the dysphagia score (above, in the patients with MSA-C; below, in the patients with MSA-P).
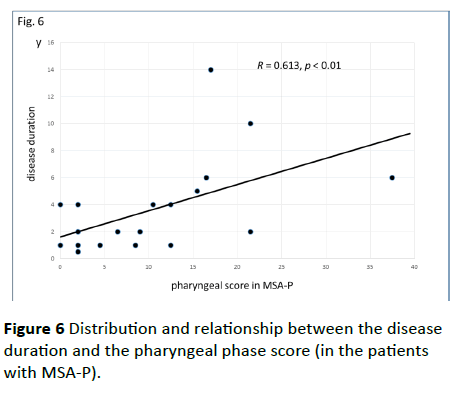
Figure 6 Distribution and relationship between the disease duration and the pharyngeal phase score (in the patients with MSA-P).
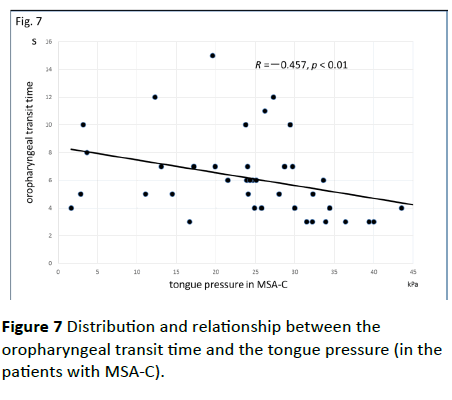
Figure 7 Distribution and relationship between the oropharyngeal transit time and the tongue pressure (in the patients with MSA-C).
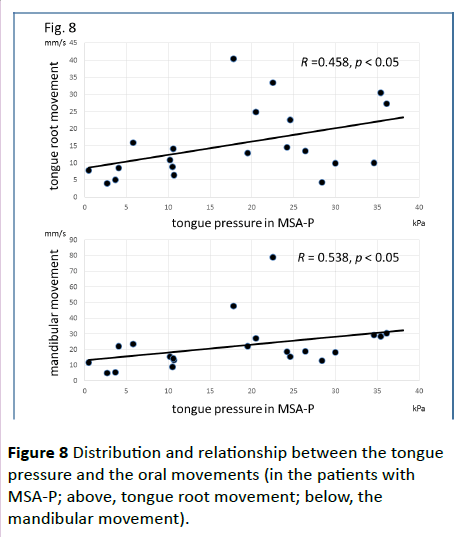
Figure 8 Distribution and relationship between the tongue pressure and the oral movements (in the patients with MSA-P; above, tongue root movement; below, the mandibular movement).
Discussion and Conclusion
This may be the first study to compare swallowing function between patients with MSA-P and those with MSA-C. From our results, patients with MSA-P tended to experience problems with propelling the bolus through the oropharynx, thus inducing a prolongation of the oropharyngeal transit time, whereas patients with MSA-C seemed to show uncoordinated bolus transportation. These findings suggested that, in the early stage of MSA, the swallowing dysfunction in patients with MSA-P that is induced by Parkinsonism is more remarkable than the dysfunction in patients with MSA-C, which is provoked by cerebellar dysfunction.
Watanabe et al. have suggested that the time from the initial symptom to the appearance of other symptoms, which indicates the evolution to MSA, is strongly related to the deterioration of ADL and a worsening of survival [3,4]. They assessed three activities of ADL milestones (aid-requiring walking, being wheelchair-bound, and being bedridden), and the time of onset of each of those states and of death in order to evaluate disease progression. A prospective European cohort study has reported that the median survival time from symptom onset was 9.8 years and patients with MSA-P were predicted to have a shorter survival [13]. However, according to the results of the study of Watanabe et al. patients with MSA-P showed more rapid functional deterioration than patients with MSA-C, but they had a similar survival time as patients with MSA-C [3]. Patients with MSA-P had more rapid functional deterioration than patients with MSA-C did. Patients with MSA-P had an accelerated risk of requiring a walking aid and a wheelchair, but the time until confinement to a bedridden state and survival was no worse. They suggested that Parkinsonism affects the motor aspects of ADL more so than cerebellar dysfunction [3]. In our MSA patients, the ADL score was significantly correlated with the dysphagia score, as well as with the tongue pressure. The disease duration was also correlated with the dysphagia score, especially with the pharyngeal phase score of MSA-P. These findings may support the relevance between the ADL deterioration and the survival time because dysphagia can have a significant impact on MSA patient’s lives.
In our MSA-P patients, the prolonged oropharyngeal transit time or the significant correlation between the movements in the oral phase and the tongue pressure may be caused by Parkinsonism, bradykinesia or hypokinesia. Alfonsi et al. reported prolongation of swallowing reaction time and reduction in duration of EMG activity of suprahyoid/submental muscles in MSA-P patients which may be explained by bradyhypokinetic mechanisms of oral swallowing [14]. Their study may be accorded with our results which suggested the impact of Parkinsonism in MSA-P on swallowing, especially in oral phase. However, MSA progression seems to influence on pharyngeal phase because of a significant correlation between the disease duration and the pharyngeal phase score in our results.
The severity of disease has been shown to be significantly correlated with a history of aspiration pneumonia. Incomplete relaxation of the upper esophageal sphincter was seen in 23.1% of patients with MSA [15]. Higo et al. have indicated that bolus transportation from the oral cavity to the pharynx is already disturbed in patients in the early stage of MSA-C [2]. The results of their study showed that 50% of the patients with MSA-C in the early stage (1−3 years following disease onset) and more than 85% in the late stage (>7 years following disease onset) suffered from delayed bolus transportation in the oral phase. It was suggested that the progression of cerebellar dysfunction and overlapping Parkinsonism worsened tongue movements [16]. In Parkinson’s disease, impaired tongue movement that is induced by Parkinsonism is the most common cause of prolonged bolus transportation [11]. The longer oropharyngeal transit time in patients with MSA-P that was observed in this study supported the view that patients with MSA-P suffer from dysphagia, which was mainly caused by Parkinsonism.
However, it is unclear whether or not cerebellar dysfunction influences swallowing function in patients with MSA-C. Although patients with MSA-C did not show a significant reduction in tongue pressure and a prolongation of oropharyngeal transit time, their oral phase scores deteriorated in direct proportion with the ADL scores. The lost points of bolus formation, mastication, or premature bolus loss in the oral phase scale in many patients with MSA-C suggest the incoordination of tongue and mandibular movements. To objectify the incoordination, further investigation is needed.
In a previous study, we studied swallowing function in mild/ moderate stage and advanced stage PD patients using the same method with this study [11]. The dysphasia score in mild/moderate PD (mild/moderate group, 13.6 ± 5.7; advanced group, 26.7 ± 19.7) and the tongue pressure in advanced PD (mild/moderate group, 32.9 ± 11.5; advanced group, 22.3 ± 13.6) were close to MSA patients. On the other hand, the tongue root movement in mild/moderate PD (mild/ moderate group, 15.4 ± 9.2; advanced group, 11.0 ± 7.2) and the mandibular movement in mild/moderate PD (mild/ moderate group, 23.0 ± 13.8; advanced group, 14.3 ± 8.8) were close to MSA patients. Although the oropharyngeal transit time in mild/moderate PD (mild/moderate group, 7.8 ± 5.9; advanced group, 12.9 ± 7.3) was close to MSA-C patients, that in advanced. PD was close to MSA-P patients. These findings suggested that the tongue pressure of the MSA patients was weakened to the level of the advanced PD patients, despite the dysphagia level and swallowing movements in the MSA patients were similar to those in the mild/moderate PD patients.
Because patients with MSA-P will show a more rapid deterioration of their swallowing function compared to patients with idiopathic Parkinson’s disease [1], we should evaluate or cope with the dysphagia in patients with MSA-P more carefully. However, very few rehabilitation programs for dysphagia have enough evidence of effective treatments, even in patients with Parkinson’s disease [17,18]. At present, frequent compensatory measures for dysphagia, such as the adjustment of diet type, liquid thickness, or swallowing posture, are more realistic strategies for maintaining the quality of life of patients with MSA-P as well as those with Parkinson’s disease [19]. However, dysphagia in patients with early stage MSA-C has been predicted to be less remarkable than in patients with MSA-P [20]. Therefore, we should first adjust the diet type for patients with early stage MSA-C to allow them to form a food bolus smoothly in the oral phase, but, in the late stage, patients with both MSA-P and MSA-C will need an ultimate alternative, such as tube feeding, in preventing aspiration pneumonia and considering the optimal timing for a gastrostomy [13].
Study Limitations
This study has some limitations which have to be pointed out. Firstly, we actually analyzed various viscosity and amount of liquids and jelly in the VF study, and ultimately adopted 5 mL each of barium water and barium gelatinous jelly by considering safeness for patients and detestability of differences between the MSA-C and MSA-P groups. Secondly, we investigated tongue pressure for some patients using both the Iowa Oral Performance Instrument [21] and the JMS tongue pressure measurement system and confirmed a significant correlation between the two systems. The both systems are possible options to assess swallowing function for MSA patients. Thirdly, a prospective European cohort study confirmed that MSA-P was more common [22], but Japanese people have higher prevalence of MSA-C than that of MSA-P [3]. The difference in sample size in this study is thought to be due to difference in prevalence. Although correction of sample size discrepancy between the two groups or larger sample size may provide a significant difference in tongue pressure, the difference in sample size is thought to have little effect on the results. Lastly, assessing dysphagia due to cerebellar dysfunction is more difficult than assessing dysphagia caused by Parkinsonism based on bradykinesia or hypokinesia, thus we must find a more accurate factor that predicts oral and pharyngeal swallowing movement.
Acknowledgments
We are grateful for the support of this work by the Japan Society for the Promotion of Science and Grants-in-aid for Scientific Research (C25463276).
Conflicts of Interest
We declare that we have no conflicts of interest.
18113
References
- Müller J, Wenning GK, Verny M, McKee A, Chaudhuri KR, et al. (2001) Progression of dysarthria and dysphagia in postmortem-confirmed parkinsonian disorders. Arch Neurol 58: 259-264.
- Higo R, Nito T, Tayama N (2005) Swallowing function in patients with multiple-system atrophy with a clinical predominant of cerebellar symptoms (MSA-C). Eur Arch Otorhinolaryngol 262: 646-650.
- Watanabe H, Saito Y, Terao S, Ando T, Kachi T, et al. (2002) Progression and prognosis in multiple system atrophy - An analysis of 230 Japanese patients. Brain 125: 1070-1083.
- Roncevic D, Palma JA, Martinez J, Goulding N, Norcliffe-Kaufmann L, et al. (2014) Cerebellar and parkinsonian phenotypes in multiple system atrophy: similarities, differences and survival. J Neural Transm (Vienna). 121: 507-512.
- Tandon R, Pradhan S (2015) Autonomic predominant multiple system atrophy in the context of Parkinsonian and cerebellar variants. Clin Neurol Neurosurg 130: 110-113.
- Gilman S, Low PA, Quinn N, Albanese A, Ben-Shlomo Y, et al. (1999) Consensus statement on the diagnosis of multiple system atrophy. J Neurol Sci 163: 94-98.
- Ohura T, Higashi T, Ishizaki T, Nakayama T (2014) Assessment of the validity and internal consistency of a performance evaluation tool based on the Japanese version of the modified barthel index for elderly people living at home. J Phys Ther Sci 26: 1971-1974.
- Hayashi R, Tsuga K, Hosokawa R, Yoshida M, Sato Y, et al. (2002) A novel handy probe for tongue pressure measurement. Int J Prosthodont 15: 385-388.
- Logemann JA (1993) Measurement of swallow from videofluorographic studies. In: Logemann JA, editors. Manual for the videofluorographic study of swallowing, (2ndedn). Pro-ed. pp. 115-126.
- Han TR, Paik NJ, Park JW, Kwon BS (2008) The prediction of persistent dysphagia beyond six months after stroke. Dysphagia 23: 59-64.
- Umemoto G, Tsuboi Y, Kitashima A, Furuya H, Kikuta T (2011) Impaired food transportation in Parkinson's disease related to lingual bradykinesia. Dysphagia 26: 250-255.
- Utanohara Y, Hayashi R, Yoshikawa M, Yoshida M, Tsuga K, et al. (2008) Standard values of maximum tongue pressure taken using newly developed disposable tongue pressure measurement device. Dysphagia 23: 286-290.
- Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, et al. (2013) The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12: 264-274.
- Alfonsi E, Versino M, Merlo IM, Pacchetti C, Martignoni E, et al. (2007) Electrophysiologic patterns of oral-pharyngeal swallowing in parkinsonian syndromes. Neurology 68: 583-589.
- Higo R, Tayama N, Watanabe T, Nitou T, Ugawa Y (2003) Videofluoroscopic and manometric evaluation of swallowing function in patients with multiple system atrophy. Ann Otol Rhinol Laryngol 112: 630-636.
- Fernagut PO, Vital A, Canron MH, Tison F, Meissner WG (2012) Ambiguous mechanisms of dysphagia in multiple system atrophy. Brain 135: 1-3.
- Baijens LW, Speyer R (2009) Effects of therapy for dysphagia in Parkinson's disease: systematic review. Dysphagia 24: 91-102.
- Miyasaki JM (2016) Treatment of advanced Parkinson disease and related disorders. Continuum (Minneap Minn) 22 (4 Movement Disorders): 1104-1116.
- Hind JA, Gensler G, Brandt DK, Gardner PJ, Blumenthal L, et al. (2009) Comparison of trained clinician ratings with expert ratings of aspiration on videofluoroscopic images from a randomized clinical trial. Dysphagia 24: 211-217.
- Tada M, Onodera O, Tada M, Ozawa T, PiaoYS, et al. (2007) Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch Neurol 64: 256-260.
- Adams V, Mathisen B, Baines S, Lazarus C, Callister R (2013) A systematic review and meta-analysis of measurements of tongue and hand strength and endurance using the Iowa Oral Performance Instrument (IOPI). Dysphagia 28: 350-369.













