Keywords
Cryopreservation; Epididymis; Histological analysis; Rat
Introduction
Add about epidydimus, its function and appearence
Cryopreservation of reproductive cells and tissues has become an increasingly important methodology for future fertility advantages [1,2]. Cryopreservation of testicular cells and tissue prior to any fertility compromising condition or therapy may allow for future tissue transplantation back to the autologous donor so that they may regain the ability to naturally conceive their own biological children [3]. Scientist regards to cryopreservation be crucial for enabling technology to the progression from preclinical and translational clinical research on cellular tissue products for regenerative medicine and transplantation. Tissue preservation is also needed for samples to be used for various research and toxicology test purposes. The need and advantages of tissue cryopreservation are widely recognized and well documented. Alternatively, these cells may be used to create new sperm outside the body through germ cell maturation protocols [4]. Investigational techniques for cryopreserving testicular tissue and cells have been tested and reported by several groups [5,6]; however, to our knowledge, a clinical-grade protocol for the cryopreservation of rat testicular cells or tissue has not been previously described.
Cryopreservation can induce production of ice crystals from the water inside the cells, which can damage the cells internal structure and cellular membrane and lead to cell death [6,7]. Studying the effect of cryopreservation on both cells and tissue will help to determine which method is most suitable and applicable for clinical use. The cells and tissue in this study were cryopreserved in a medium using cryoprotectants to prevent ice crystals from forming, thus improving the ability of the cells to survive freezing and thawing. Testicular tissue cryopreservation would be an important technique for fertility preservation who do not yet have sperm in the ejaculate and who are scheduled to undergo gonadotoxic treatment [8]. Cryopreservation of semen and spermatozoa is a welldeveloped technique, routinely used in everyday practice in infertility laboratories.
Several descriptive anatomical and histological studies of the epididymis appeared at the beginning of the twentieth century. Since 1966, more than 12,000 research articles have been published on the epididymis. While addressing the various aspects of the epididymis from several points of view, all agree that the epididymis is crucial for the preparation of the spermatozoa prior to ejaculation. It is well known that the proteins and small molecules secreted by the epididymal epithelium into the lumen interact with the transiting spermatozoa and directly or indirectly affect the spermatozoa surface; these epididymal proteins and small molecules have many other functions other than signaling inducing protein activity [9]. A significant number of these molecules will be gradually rearranged and compartmentalized in a stagespecific manner during sperm maturation [10] (Figure 1).
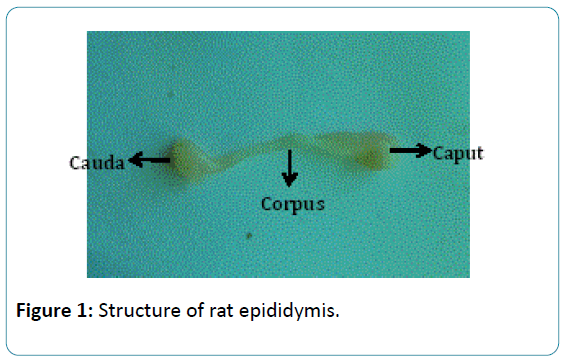
Figure 1: Structure of rat epididymis.
The mammalian epididymis is no longer regarded as a mere conduit pipe for spermatozoa from the testis to the exterior [11]. The epididymis as an epithelial tube is folded into a highly organised structure comprised of many segments that can be grouped into roughly five gross anatomical segments, namely: caput, corpus and cauda with each having distinct morphology and function [12]. So, in assessing the contributions of the different segments in providing an appropriate environment for sperm maturation, the knowledge of its structure is vital.
The spermogram accurately assesses male fertility through an evaluation of semen quality, sperm concentration, motility and morphology [13]. These men may present with posttesticular defects that result in the ejaculation of spermatozoa with a normal morphology but with a sub-optimal fertilization capacity [14].
Therefore the epididymis could be particularly involved in a number of the pathophysiologies affecting sperm maturation in some of these cases of male infertility [13,14]. A valuable approach is represented by the cryopreservation of the micro surgically recovered spermatozoa, thus avoiding a second surgical operation for further ICSI attempts. Indeed, pregnancies and deliveries have been reported after injection of frozen–thawed epididymal [15] and testicular spermatozoa [16].
Therefore, to describe the histological appearance of the epididymis in the greater rat was selected (Rattus norvegicus). The data might provide the basis for future research as well as contribute to the knowledge of the reproductive biology of rat.
Materials and Methods
Cryopreservation of epididymis
Male rats were euthanized via ethanol inhalation followed with induction of bilateral pneumothorax. Immediately following confirmation of sedation, the distal end of the scrotal sac was removed and dissection of the tunics allowed for exteriorization of the testis and cauda epididymidis. The cauda epididymidis without fat was removed from the testicle and was rinsed in warm phosphate buffered saline (DPBS). Later using scissors it was cut into three slits. The epididymis was then placed in the 4ml cryovial containing selected cryopreservation medium (1.0:0.7 (w:v) ), i.e. Freeze™, a 15% glycerol based cryoprotectant in HEPES buffer (Ferti Pro N.V., 8730 Beernem, Belgium). This tissue was subjected to static vapour phase cooling for 15min before being plunged into liquid nitrogen. Tissues were subsequently thawed at 37°C for 10min by placing the cryovials in waterbath and then subjected to histomorphological analysis. This study was authorized by a institutional animal ethical committee (Reg. No.438/01/01a/Dt:17-07-2001) and resolution number is 40/2012-2013(i)/a/CPCSEA/IAEC/SVU/KTR-KK.
Preparation of slides for histopathology
The frozen-thawed rat epididymidal tissue of fresh (control), 15, 30, 45 and 60 days were dehydrated through a graded series of alcohols, the tissues were cleared in methyl benzoate and embedded in paraffin wax. Sections were cut at 6μ thickness and stained with haematoxylin [17] and counter stained with Eosin dissolved in 70% alcohol. After dehydration and cleaning, sections were mounted in Canada balsam. Histological examinations of the tissues were followed according to Humason [18] and the specimens were observed under the light microscope. Photomicrographs were taken by Ricoh 35 mm SLR camera.
Results and Discussion
Cryobiology is defined as the study of the subjects under effects of temperatures lower than normal physiologic ranges upon biological systems [19]. Simple cooling of cells or tissues with spontaneous ice nucleation and crystal growth shall results in dead or nonfunctional mass. Histology is an important way of studying the anatomical variations of tissues in the rat under the influence of different insults which may contain cryopreservation as one of the factors. The present studies have observed many changes in histological preparations of the frozen samples compared with the fresh group.
To the earlier studies on sperm biochemical parameters of our laboratory [20] this study revealed that the epididymis of the fresh and frozen-thawed groups had histological variations studies as represented in Figures 2-6, and the epididymis of the fresh and frozen-thawed samples histology showed full and less of spermatozoa in lumen, respectively (Figures 2-6).
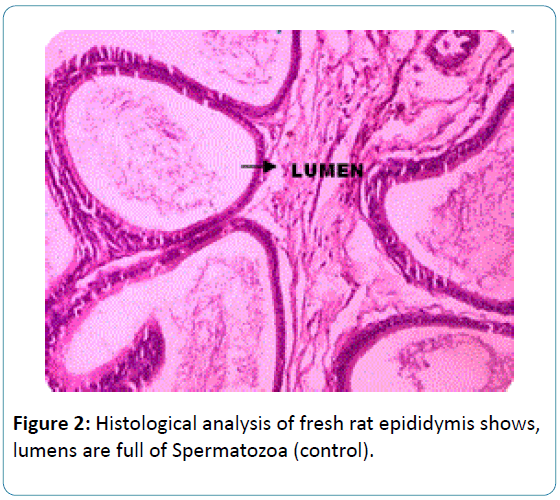
Figure 2: Histological analysis of fresh rat epididymis shows, lumens are full of Spermatozoa (control).
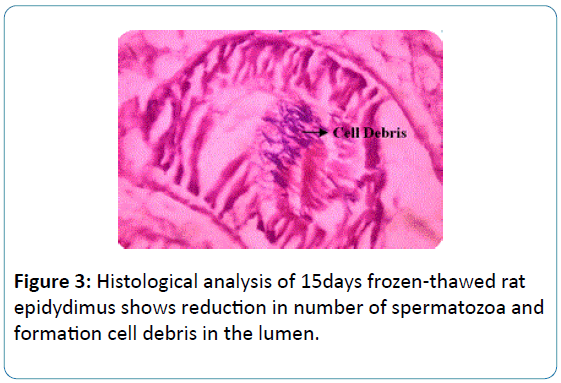
Figure 3: Histological analysis of 15days frozen-thawed rat epidydimus shows reduction in number of spermatozoa and formation cell debris in the lumen.
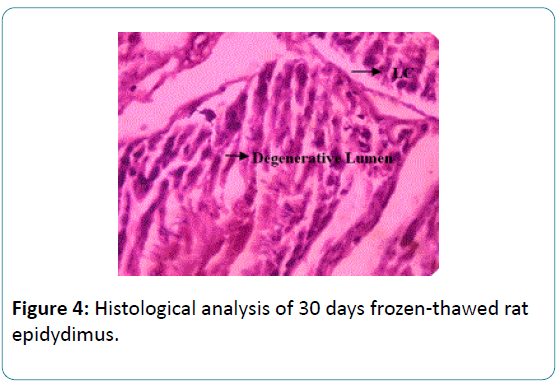
Figure 4: Histological analysis of 30 days frozen-thawed rat epidydimus.
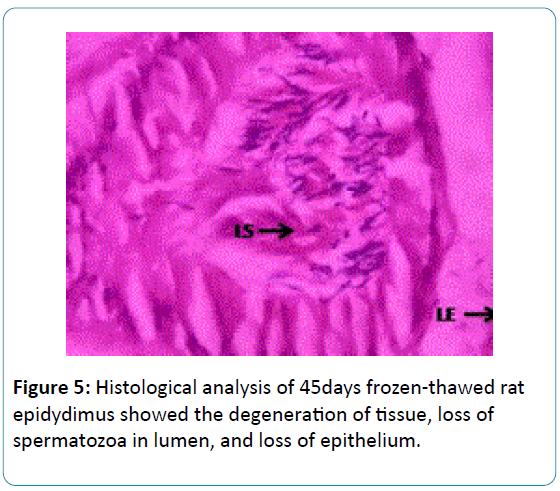
Figure 5: Histological analysis of 45days frozen-thawed rat epidydimus showed the degeneration of tissue, loss of spermatozoa in lumen, and loss of epithelium.
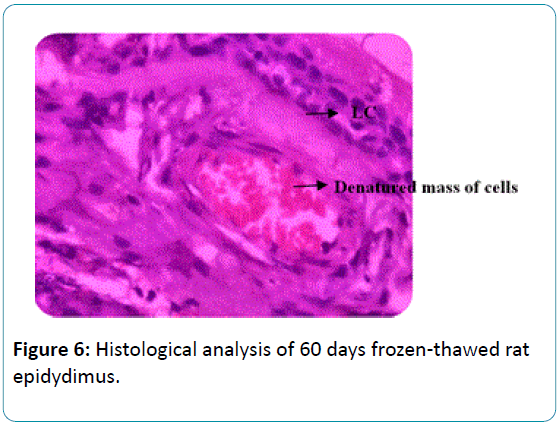
Figure 6: Histological analysis of 60 days frozen-thawed rat epidydimus.
The use of cryotechniques could generate histological changes as observed in images presumed due to the formation of large crystals of ice that, in turn, may alter or even destroy the structure of the cell or tissue [21]. In the present study, after 15 days of cryopreservation of rat epidydimis a great reduction in number of sperm in lumen due to crystal formation in the cells Figure 3.
The 30 days cryopreserved rat epidydimis were showed a significant reduction in number of spermatozoa and presence of cell debris in the lumen. It also shows degenerative changes in tissue with elongated tubules and loss of connective tissue Figure 4.
In the 45 days cryopreserved rat epidydimis had formed the degeneration of tissue, distractive changes in lumen, epithelium and loss of connective tissue, which were shown in the Figure 5.
Finally, the 60 days cryopreserved rat epididymis showed the degeneration of tissue which indicates structural changes in lumen, epithelium and loss of connective tissue, the lumen of epidydimus shows masses of cells which appeared to be that denatured protein material of spermatozoa Figure 6.
Cryobiology has explained cryoprocessing of tissue and their samples has several advantages, especially the maintenance of the structure and biochemical composition of the tissue [22]. In this milieu, handling and processing of production and using cryopreservatives could be a difficult technique [23], and no works have been reported on the use of this histology method on rat epidydimal tissues. An optimized amount of gonadal tissue for freezing is an important issue [24,25]. However, it is important to inform all patients facing infertility as a side effect of gonadotoxic chemo- and radio-therapies about the options available to preserve their future fertility [26,27]. Previously, a comparative study was performed in which cryopreservation of human ovarian tissue was carried out with cryoprotectant media [28]. Taking into consideration these results in our experiments, the rat epidydimus used for cryopreservation.
The use of cryotechniques could generate histological images mainly due to the formation of large crystals of ice that, in turn, may alter or even destroy the structure of the tissue. To avoid the formation of large ice crystals, the use of certain cryoprotective agents has been suggested [29-31]. For that reason, in this work, we have carried out cryopreservation with 15% glycerol. Different substances have been used so far as cryoprotective solutions prior to cryofixation of native tissues [20,31] especially sucrose [32,33] and trehalose [34].
In the male reproductive system, the testis is the source of hormones, testicular proteins, and spermatozoa, while the epididymis is the organ responsible for maturation, storage, and survival of male gametes. The full fertilizing ability of released spermatozoa is dependent on series of morphological, biochemical, and functional changes during the transit through the epididymis. In the epididymis, the numbers of sperm were greatly reduced or absent from the lumen of the severely affected animals. Since epithelium did not appear to be significantly altered in its complement of epidydimus and there were no indications of infiltration of phagocytic cells, the lack of sperm seems most likely due to the cold shock effects of cryopreservation. The presence of material in lumen of epididymis on the basis of light microscope observed as the degeneration of tissue which indicates structural changes in lumen, epithelium and loss of connective tissue, the lumen of epidydimus shows masses of cells which appear to be that denatured protein material of spermatozoa. Reports were available on the histology of the epididymis in adults of different species but very few reports were available on the histology after cryopreservation.
Compared to the fresh and frozen tissue had to some disturbed morphology, probably because of recovery of the tissue after cold temperature shock and the handling procedure. Mostly, low survivals of sperm cells were seen. In fact, histological comparison of the rat epidydimides of fresh and frozen tissues preserved for 15, 30, 45 and 60 days show significant differences. Strikingly, cryopreservations of the samples for 15 days resulted greatly reduce in number of sperm in lumen of epididymis due to the effect intra cellular ice formation in the cells. Similarly, the 30, 45 and 60 days frozen- thawed resulted in disturbed tissue quality, with degeneration of tissue which indicates structural changes in lumen, epithelium and loss of connective tissue, the lumen of epidydimus shows masses of cells which appear to be that denatured protein material of spermatozoa were observed when compared with control. Finally, the results of the tissue cryopreserved having more frozen effects on the epidydimides and the spermatozoa embedded in tissue were also disturbed with incubation time.
Accordingly, we can conclude that the cryopreservation causes severe intracellular damage to epidydimal tissue. However, our results suggest that freezing thawing of the spermatozoa with highly expertise technician is more useful than epidydimus cryopreservation. For all those reasons, we would recommend the use semen suspensions cryopreservation is effective than tissue cryopreservation except in the case of ejaculatory dysfunction.
8942
References
- Orwig KE, Schlatt S (2005) Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr : 51-56.
- Wyns C, Curaba M, Vanabelle B, Van Langendonckt A, Donnez J (2010) Options for fertility preservation in prepubertal boys. Hum Reprod Update 16: 312-328.
- Brook PF, Radford JA, Shalet SM, Joyce AD, Gosden RG (2001) Isolation of germ cells from human testicular tissue for low temperature storage and autotransplantation. Fertil Steril 75: 269-274.
- Stukenborg JB, Schlatt S, Simoni M (2009) New horizons for in vitro spermatogenesis? An update on novel three dimensional culture systems as tools for meiotic and post meiotic differentiation of testicular germ cells. Mol Hum Reprod 15: 521-529.
- Brinster RL, Nagano M (1998) Spermatogonial stem cell transplantation, cryopreservation and culture. Semin Cell Dev Biol 9: 401-409.
- Keros V, Rosenlund B, Hultenby K, Aghajanova L, Levkov L, et al. (2005) Optimizing cryopreservation of human testicular tissue: comparison of protocols with glycerol, propanediol and dimethylsulphoxide as cryoprotectants. Hum Reprod 20: 1676-1687.
- Pegg DE (2010) The relevance of ice crystal formation for the cryopreservation of tissues and organs. Cryobiology 60: S36-44.
- Hovatta O (2001) Cryopreservation of testicular tissue in young cancer patients. Hum Reprod Update 7: 378-383.
- Gatti JL, Castella S, Dacheux F, Ecroyd H, Métayer S, et al. (2004) Post-testicular sperm environment and fertility. Anim Reprod Sci 82-83: 321-39.
- França LR, Avelar GF, Almeida FF (2005) Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 63: 300-318.
- Reid BL, Cleland KW (1957) The structure and function of the epididymis. The histology of the rat epididymis. Aus J Zool 5: 223-246.
- Joseph A, Yao H, Hinton BT (2009) Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev Biol 325: 6-14.
- Hamada A, Esteves SC, Agarwal A (2011) Unexplained male infertility: potential causes and management. Review article. Human Androloly 1: 2-16.
- Sullivan R (2004) Male fertility markers, myth or reality. Anim Reprod Sci 82-83: 341-7.
- Devroey P, Silber S, Nagy Z, Liu J, Tournaye H, et al. (1995) Ongoing pregnancies and birth after intracytoplasmic sperm injection with frozen-thawed epididymal spermatozoa. Hum Reprod 10: 903-906.
- Gil-Salom M, Romero J, Minguez Y, Rubio C, De los Santos MJ, et al. (1996) Pregnancies after intracytoplasmic sperm injection with cryopreserved testicular spermatozoa. Hum Reprod 11: 1309-1313.
- Harris HR (1900) On the rapid conversion of haematoxylin into haematein in staining reactions. J Appl Microsc 3: 777-780.
- Humason GL (1962) Animal tissue techniques-3rd Ed, San Fransisco: W.H. Freeman. USA.
- Kelvin G.M. Brockbank and Michael J. Taylor (2006) Tissue Preservation. Advances in Biopreservation 157-196.
- POLGE C, SMITH AU, PARKES AS (1949) Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature 164: 666.
- Thyaga Raju K, Kamala K, Divya K (2014). DNA damage in sperm due to cryopreservation- A study in rats. World Journal of Pharmaceutical Research 3:1676-1683.
- Di Santo M, Tarozzi N, Nadalini M, Borini A (2012) Human Sperm Cryopreservation: Update on Techniques, Effect on DNA Integrity, and Implications for ART. Adv Urol 2012: 854837.
- Tournaye H, Goossens E, Verheyen G, Frederickx V, De Block G, et al. (2004) Preserving the reproductive potential of men and boys with cancer: current concepts and future prospects. Hum Reprod Update 10: 525-532.
- Franks F(1989) Improved freeze-drying: an analysis of the basic scientific principles. Process Biochem 24: 3-8.
- Jahnukainen K, Ehmcke J, Söder O, Schlatt S (2006) Clinical potential and putative risks of fertility preservation in children utilizing gonadal tissue or germline stem cells. Pediatr Res 59: 40R-7R.
- Robertson JA (2005) Cancer and fertility: ethical and legal challenges. J Natl Cancer Inst Monogr 34: 104-106.
- Wallace WH1, Anderson RA, Irvine DS (2005) Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol 6: 209-218.
- Hreinsson J, Zhang P, Swahn ML, Hultenby K, Hovatta O (2003) Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum Reprod 18: 2420-2428.
- Barthel LK, Raymond PA (1990) Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem 38: 1383-1388.
- Naber SP, Smith LL Jr, Wolfe HJ (1992) Role of the frozen tissue bank in molecular pathology. Diagn Mol Pathol 1: 73-79.
- Loken SD, Demetrick DJ (2005) A novel method for freezing and storing research tissue bank specimens. Hum Pathol 36: 977-980.
- Ljungberg A, Johansson O (1993) Methodological aspects on immunohistochemistry in dermatology with special reference to neuronal markers. Histochem J 25: 735-745.
- Whitlon DS, Szakaly R, Greiner MA (2001) Cryoembedding and sectioning of cochleas for immunocytochemistry and in situ hybridization. Brain Res Brain Res Protoc 6: 159-166.
- Norville JE, Kelly DF, Knight TF Jr, Belcher AM, Walz T (2007) 7A projection map of the S-layer protein sbpA obtained with trehalose-embedded monolayer crystals. J Struct Biol 160: 313-323.











