Keywords
Iron deficiency anaemia, Diabetes, Haemoglobin A1c
Introduction
Haemoglobin A1c (HbA1c) is a glycated haemoglobin that is used as an indicator of a patient’s glycemic status over the previous three months [1]. According to the recent American Diabetes Association Guidelines, HbA1c levels should be maintained below 7% in all diabetic patients in order to prevent the development of microvascular complications [2]. Also as per the recent recommendations by the IDF and AACE, the optimum level at which HbA1c should be maintained in diabetics has been brought down to the target of 6.5%. HbA1c levels are not affected by blood glucose alone. They are also altered in haemolytic anaemias [3], haemoglobinopathies [4], acute and chronic blood loss [5,6], pregnancy [7-9] and uremia [10-12]. Vitamin B12, folate and iron deficiency anaemia have also been shown to affect HbA1c levels.
Iron deficiency anaemia is the most common form of anaemia observed in Indian settings [13] and India being the diabetes capital of the world, it was imperative to find out the relationship that exists between iron deficiency anaemia and HbA1c which is one of the commonest investigation carried out routinely in diabetic patients. Initial studies done on the association of Iron deficiency anaemia with HbA1c levels by Brooks et al. [14]. Sluiter et al. [15], and Mitchell et al. [16]. revealed a relationship between them existed and attempted to explain the alteration in HbA1c levels in iron deficiency anaemia on the basis of both modifications to the structure of haemoglobin and levels of HbA1c in old and new red blood cells. Later, Heyningen et al. [17] and Hansen et al. [18] reported that there were no differences between the HbA1c levels of anaemic patients and controls. Rai et al. [19] investigated different methods to assay HbA1c levels and found no differences in HbA1c levels detected when using calorimetric assays, ion exchange chromatography, and affinity chromatography. An Indian study done by Nitin et al. [20] came out with the results that iron deficiency anaemia and HbA1c levels were directly proportional and both of them increased or decreased in the same direction.
The matter of the fact is that this entity called HbA1c is not just about diabetes and blood glucose control, rather it is affected by multiple factors. Iron deficiency anaemia being a very common comorbidity found in Indian population itself impacts a person’s HbA1c level and its correction bring its level back to normal. So it becomes prudent to show the effect of iron deficiency anaemia on HbA1c levels before any decision or guidelines are made based on HbA1c levels.
The results of all studies done previously on this topic are conflicting, and the exact mechanism as well as relationship between iron deficiency anaemia on HbA1c levels is not yet known. Therefore, both because of this lack of corresponding evidence and since no conclusive studies exist for this topic, we were prompted to conduct the current study to investigate the effects of Iron Deficiency Anaemia on HbA1c levels in Indian patients.
Methods
50 confirmed cases of iron deficiency anaemia with Haemoglobin <12 mg/dl in women and <13 mg/dl in men were included in study group and Iron deficiency was confirmed in them by serum Ferritin levels <30 ng/dl and serum Iron levels <65 μg/dl in males and <50 μg/dl in females. Patients with history of acute blood loss, haemolytic anaemia, haemoglobinopathies, kidney disease, pregnancy, established diabetes, impaired fasting glucose or impaired glucose tolerance were excluded from the study. Even patients with no history of glucose intolerance but with fasting blood glucose >100 mg/dl at the time of enrolment were excluded. Women of child bearing age who have amenorrhoea and those with reticulocyte count more than 2.5 or blood urea greater than 40 were also excluded from the study. 50 healthy and matched controls were included for comparison. However, exclusion criteria for controls was same as that for patients. All the patients enrolled in the study were from outdoor and indoor departments of Vivekananda Polyclinic and Institute of Medical Sciences, Lucknow and a written informed consent was taken from all before enrolment.
All patients were subjected to a detailed history and physical examination. Splenomegaly was graded as mild (<2 cm), moderate (2 cm to 7 cm) and severe (>7 cm). Haemoglobin, MCV, MCH, MCHC, haematocrit, platelet count, TLC, DLC, ESR and peripheral smears were examined at the time of enrolment (baseline) and at the end of three months the second time.
Based on haemoglobin values, patients were termed as anaemic with cut off of <13 mg/dl in men and <12 mg/dl in women. Reticulocyte count was measured at baseline and reticulocyte production index was calculated. If patients are found to have reticulocyte production index >2.5 at baseline in the absence of overt bleeding were considered to be having haemolytic anaemia and were then excluded. Those with predominantly microcytic (MCV<80 fentolitre) and hypochromic (MCH<26 picogram/cell) indices were considered to be having iron deficiency anaemia which was confirmed by measuring serum iron and serum ferritin. Those who had serum ferritin <30 ng/dl and serum iron <65 μg/dl (males) and <50 μg/dl (female) were confirmed to have iron deficiency. Serum ferritin and serum iron were measured again after 3 months to see the difference in values after correction of anaemia with Iron replenishment. Serum ferritin was measured by an ELISA test kit (Biochek Inc. India). HbA1c was measured for the study group patients at the time of enrolment (baseline) and after 3 months of iron correction therapy. HbA1c levels were measured using the glycohaemoglobin reagent kit (TECO Diagnostics India) which works on the principle of exchange column chromatography. Fasting blood sugar levels were measured at the beginning and then at 3 months of treatment to exclude diabetes or impaired glucose tolerance. Those with fasting blood sugar less than 100 mg/dl were included in the study. Also done was urine pregnancy test in females to rule out pregnancy. KFT’s were done at baseline to rule out renal failure.
All patients were treated with oral ferrous sulphate (195 mg in each tablet) in adequate doses as per the severity of anaemia. Those in control group were subjected to Haemoglobin, MCV, MCH, MCHC, TLC, DLC, ESR, platelet count, haematocrit, fasting blood glucose levels, serum ferritin and HbA1c measurements along with peripheral smear examination at the time of enrolment only once in the research period. Kidney function test and fasting blood glucose were also done for the control group. The data was presented as mean (SD) for continuous variables. A student’s test was applied for comparison of group means. Pearson’s coefficient of correlation was calculated to find correlation between two variables. A p<0.05 was considered statistically significant.
Results
Of the 50 patients in study group, there were 33 females (66%) as compared 20 (40%) in the controls. This suggests that iron deficiency is commoner among females. The mean age of patients in study group was 30.3 years as compared to 36.24 years in controls. The minimum age in patients in study group was 12 years and maximum age was 50 years. The minimum age in controls was 13 years and maximum was 50 years.
Of all the symptoms in patients of study group, weakness was seen in 48 (96%), malaise in 45 (90%), disinterest in work in 30 (60%), dyspnoea was present in 19 (38%), pica in 4(8%) and 4 (8%) patients gave history of passage of worms in stools. Asking questions on overt bleeding revealed that 11 (22%) out of 33 female’s patients had menstrual complaints out of which menorrhagia was the most prevalent, being present in 10 (91%) out of 11 patients. 11 (22%) patients had bleed from other sites of which piles was the most prevalent cause, being present in 10 (91%) patients. Dietary pattern in iron deficiency anemia patients revealed 34 (68%) patients to be vegetarians and the remaining 16 (32%) to be non-vegetarians. This observation goes on to suggest that iron deficiency is seen more commonly among vegetarians.
Pallor as a sign was present in all the patients. Lymphadenopathy was seen in 1 (2%) patient which on FNAC was suggestive of reactive lymphoid hyperplasia. Hepatomegaly was seen in 1 (2%) patient who had a sigmoid mass. Splenomegaly was seen in 12 (24%) patients and all of them had mild splenomegaly (<2 cm); nail changes were seen in 6 (12%) patients out of which 5 (84%) had platynychia and 1 (16%) had koilonychia. This suggests that platynychia is more frequently seen than koilonychia in patients of iron deficiency anemia, otherwise, nail changes are not that common. Three (6%) patients had an ejection systolic murmur at apex probably related to the hyperdynamic state seen in anemia patients.
The mean hemoglobin value (g/dl) of patients at baseline was 6.18 (SD=2.12) which was significantly lower than the mean hemoglobin value of controls 13.09 (SD=0.70) (p<0.01). The mean hemoglobin inpatients rose to 12.6 (SD=1.01) after 3 months of treatment but was still lower than the control value and this difference was still statistically significant (p<0.01).
The rise in hemoglobin value from baseline to the 3 months’ value was highly significant (p<0.01). This goes to suggest that patients responded well to the treatment but were unable to achieve the control values in the 3 months duration of treatment.
The mean serum ferritin values in patients of study group at baseline was 7.51 (SD=2.49) ng/ml and that in controls at the baseline was 221.03 (SD=91.92). This difference between patients and controls was highly significant (p<0.01).
The mean ferritin value in patients rose to 279.09 (SD=83.78) which was highly significant (p<0.01). The mean serum ferritin value of patients at 3 months’ period was higher than that of controls and this difference was also significant (p<0.0L). The rise in serum ferritin on treatment was as expected (51) indicating increased availability of iron and increasing iron stores.
Mean serum iron levels at baseline in study group was found to be 15.29 mg/dl (SD=5.01) while at 3 months after treatment these levels rose to 112.74 mg/dl (SD=14.64). This signifies that with treatment of anaemia in these 3 months, the iron levels had increased markedly. Serum iron levels in controls was found to be 91.92 mg/dl (SD=20.69).
The mean HbA1c value (%) in patients of study group at baseline was 6.60 (SD=0.18) which was significantly higher than that of controls, 5.48 (SD=0.56) at the baseline (p<0.0l). However, at 3 months, the mean HbA1c (%) value of 5.74 in patients was significantly higher than that of controls (p<0.01). There was a significant decline in the mean HbA1c (%) value, 5.74 after 3 months, from the baseline value of 6.60 after treatment (p<0.01).
There was a significant correlation observed between hemoglobin values and mean HbA1c value both at baseline (Coefficient of correlation= -0.813, p<0.01) and after 3 months of treatment (coefficient of correlation= -0.296: p=0.003) in study group patients (Figure 1). These observations suggest that the HbA1c values decreased along with the corresponding increase in hemoglobin values in the 3 months of treatment which was significantly correlated with each other.
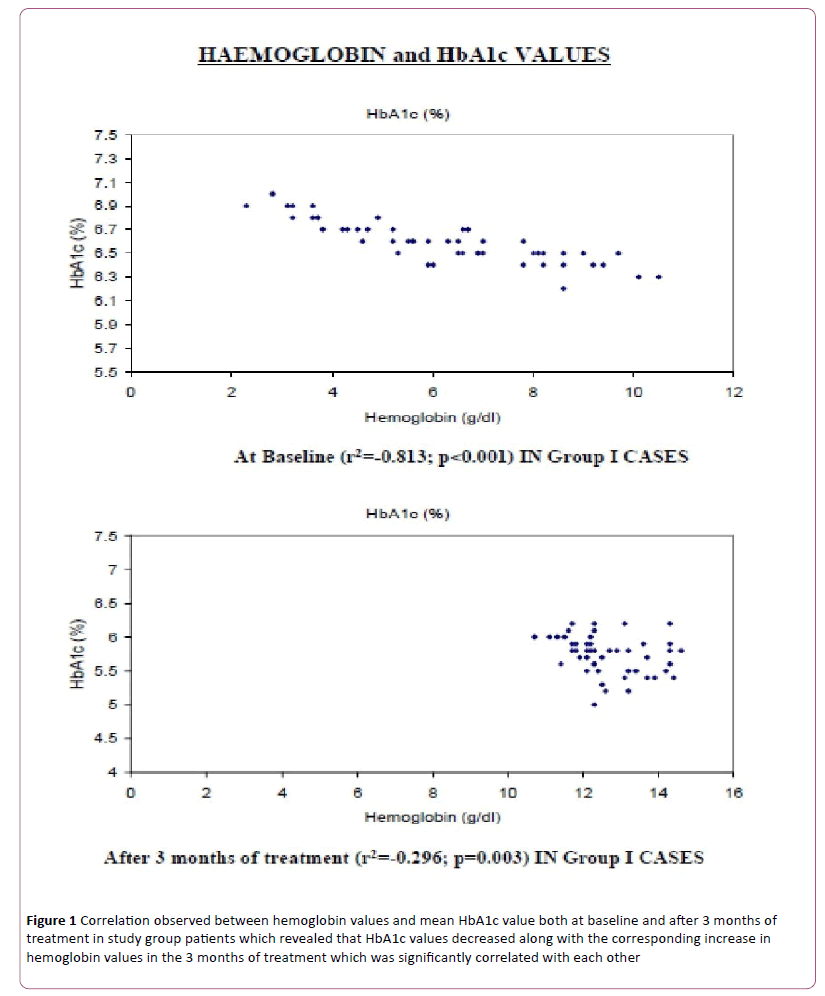
Figure 1: Correlation observed between hemoglobin values and mean HbA1c value both at baseline and after 3 months of treatment in study group patients which revealed that HbA1c values decreased along with the corresponding increase in hemoglobin values in the 3 months of treatment which was significantly correlated with each other
On comparing the two groups of study group cases (I) and controls (II) it was found that the variables of reticulocyte count, ESR, HbA1c and FBS were higher in group (I) of cases which were our iron deficiency anaemic patients.
The other variables of haemoglobin, haematocrit, MCV, MCH, MCHC, TLC, platelet count, S. ferritin and S. iron were found to be comparatively higher in group II of controls which consisted of apparently normal subjects (Table 1).
| |
Group I(n=50)
Cases |
Group II(n=50)
Controls |
Statistical
Significance |
| Mean |
SD |
Mean |
SD |
't' |
'P' |
| Haemoglobin |
6.18 |
2.12 |
13.09 |
0.70 |
-21.857 |
<0.001 |
| Het |
19.34 |
6.60 |
37.36 |
3.08 |
-17.511 |
<0.001 |
| MCV |
67.64 |
4.98 |
91.56 |
3.85 |
-26.857 |
<0.001 |
| MCH |
21.91 |
2.76 |
32.41 |
1.84 |
-22362 |
<0.001 |
| MCHC |
31.90 |
3.16 |
35.40 |
1.94 |
-6.676 |
<0.001 |
| TLC |
7952.00 |
2559.14 |
7976.00 |
2150.99 |
-0.051 |
0.960 |
| Plateletcount |
2.25 |
0.94 |
2.40 |
0.67 |
-0.934 |
0.353 |
| Retiecount |
3.57 |
1.22 |
1.39 |
0.44 |
11.933 |
<0.001 |
| ESR |
14.30 |
5.33 |
11.92 |
4.22 |
2.474 |
0.015 |
| Ferritin |
7.51 |
2.49 |
221.03 |
75.74 |
-19.924 |
<0.001 |
| S.iron |
15.29 |
5.01 |
91.92 |
20.69 |
-25A59 |
<0.001 |
| HbA1C |
6.60 |
0..1S |
5.48 |
0.56 |
13.485 |
<0.001 |
| FBS |
88.86 |
5.91. |
87.34 |
5.56 |
1.317 |
0.191 |
Table 1: Comparison of hematoloecali biochemical variables between cases and controls at baseline.
Difference between the above two groups was found to be statistically significant for all the variables except TLC, platelet count and FBS.
These findings signify that when iron deficient anaemic subjects are compared with apparently normal controls, they are found to have lower levels of haemoglobin, haematocrit, MCV, MCH, MCHC, S. ferritin, S. iron but higher levels of reticulocyte count, ESR, HbA1c and this conclusion was even statistically significant (Figure 2).
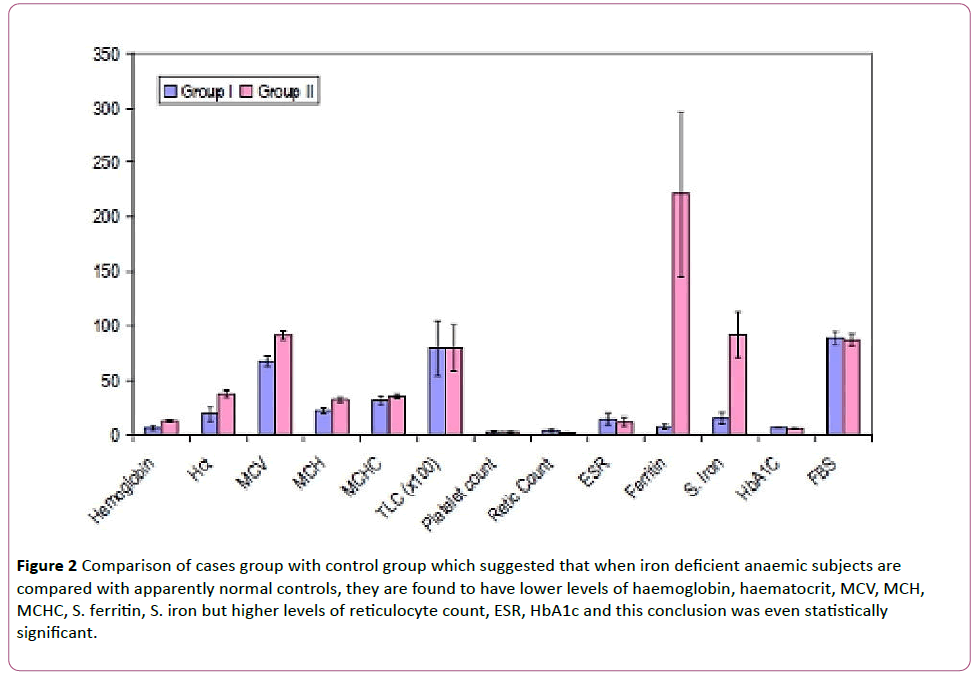
Figure 2: Comparison of cases group with control group which suggested that when iron deficient anaemic subjects are compared with apparently normal controls, they are found to have lower levels of haemoglobin, haematocrit, MCV, MCH, MCHC, S. ferritin, S. iron but higher levels of reticulocyte count, ESR, HbA1c and this conclusion was even statistically significant.
The group I of cases with iron deficiency anaemia was treated for 3 months with iron replacement therapy and again evaluated after those 3 months for change in various parameters. It was observed that almost all variables underwent significant change in values. Haemoglobin increased from 6.18 (SD=2.12) to 12.60 (SD=1.01). Haematocrit increased from 19.34 (SD=6.60) to 37.59 (SD=3.58). The Blood indices also changed with MCV from 67.64 (SD=4.98) to 92.33 (SD=3.25), MCH from 21.91 (SD=2.76) to 31.11 (SD=1.56) and MCHC from 31.90 (SD=3.16) to 33.51 (SD=1.27). S. ferritin levels increased from 7.51 (SD=2.49) to 279.08 (SD=83.87) and S. iron increased from 15.29 (SD=5.01) to 112.74 (SD=14.64) as a result of the iron replacement therapy given in these 3 months (Table 2) However, the variable under study which was HbA1c decreased to normal levels with treatment of iron deficiency in these anaemic subjects with its value decreasing from 6.60 (SD=0.18) to 5.74 (SD=0.28). All these changes in the important variables were found to be statistically significant (Figure 3).
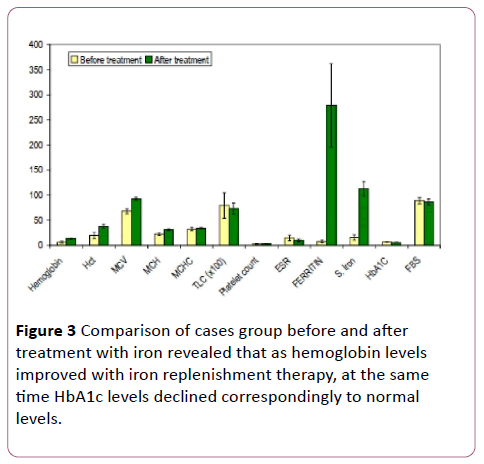
Figure 3: Comparison of cases group before and after treatment with iron revealed that as hemoglobin levels improved with iron replenishment therapy, at the same time HbA1c levels declined correspondingly to normal levels.
| |
Beforetreatment
GroupICases |
Aftertreatment
GroupICases |
Statisticalsignificance
(Paired"t"test) |
| Mean |
SD |
Mean |
|
SD |
|
| Hemoglobin |
6.18 |
2.12 |
12.60 |
1.01 |
28.701 |
<0.001 |
| Het |
19.34 |
6.60 |
37.59 |
3.58 |
25.656 |
<0.001 |
| MCV |
67.64 |
4.98 |
92.33 |
3.25 |
31.082 |
<0.001 |
| MCH |
21.91 |
2.76 |
31.11 |
1.56 |
23.401 |
<0.001 |
| MCHC |
31.90 |
3.16 |
33.51 |
1.27 |
3.683 |
0.001 |
| TLC |
7952.00 |
2559.44 |
7280.00 |
1153.70 |
1.818 |
0.075 |
| Plateletcount |
2.25 |
0.94 |
154 |
0.55 |
2.920 |
0,00:5 |
| ESR |
14.30 |
5.33 |
9.48 |
2.62 |
6.069 |
<0.001 |
| Ferritin |
7.51 |
2.49 |
279.08 |
83.78 |
22.913 |
<0.001 |
| S.iron |
15.29 |
5.01 |
11174 |
14.64 |
46.032 |
<0.001 |
| HbA1C |
6.60 |
0.18 |
5.74 |
0.28 |
19.917 |
<0.001 |
| FBS |
88.86 |
5.98 |
86.18 |
5.87 |
2.304 |
0.026 |
Table 2: Incidence of events over the 20-year period within the two groups of patients.
Discussion
Iron deficiency anemia is most common form of anemia observed in our country. HbA1c is one of the glycated haemoglobins which is used to assess the glycemic status of an individual over last 2 to 3 months and is mostly being used in diabetics and in those with impaired glucose tolerance. Certain studies have been done which show that HbA1c levels are affected in haemolytic anemias. In one of these studies done by Horton and Huisman [3] showed that HbA1c is decreased due to the reason that the life span of the RBC's is reduced. So, from these studies it became evident that HbA1c should be taken as a measure of glycemic control only if such disorders are ruled out. However, interest further arose as to what happens to HbA1c levels in more commonly encountered anemias like iron deficiency anemia. Brooks et al. [14] showed that HbA1c levels were higher in patients of iron deficiency anemia at baseline and decreased on treatment. The reason speculated by them was that the quaternary structure of haemoglobin gets altered and that, glycation of beta globin chain occurs more readily in the relative absence of iron. Sluiter et al. [15] later gave a different reason to explain the findings of Brooks et al. They were of the view that the formation of glycated hemoglobin is an irreversible process and hence, the concentration of HbA1 in one erythrocyte will increase linearly with the cell's age. In patients with normal blood glucose values but with red cells that are younger than usual, as after treatment of iron deficiency anaemia, HbA1 concentration falls. However, if the iron deficiency has been persisting for a long time, the red cell production rate falls, leading not only to anemia but also to a higher than normal average age of circulating erythrocytes and, therefore, of increased HbA1. Mitchell et al. [16] commented on the study done by Brooks et al. and also on the reasoning of Sluiter et al. From the values available in Brooks study, they rather than taking HbA1c (%), calculated mean corpuscular hemoglobin HbA1c i.e. amount of HbAlc per cell and observed that though the percentage of HbA1c decreased during treatment, MCHbA1c remained relatively constant and there was no significant difference noted between baseline and post treatment values. They were also of the view that red cell age as proposed by Sluiter et al, was unlikely to explain the changes observed in HbA1c (%) in the Brooks study. Heyningen et al. [17] demonstrated that there was no difference seen in HbA1c (%) values at baseline and after treatment in iron deficiency anemia patients and speculated that the differences observed previously could be due to different methods used in calculating HbA1c. But Rai et al. [19], reported that there was no significant difference in HbA1c values calculated by colorimetry, ion exchange chromatography and affinity chromatography. Hansen et al. [18] showed that there was no difference in HbA1c values at baseline between iron deficiency anaemia patients and controls but demonstrated a fall in HbA1c levels after treatment which they explained by stating that it was due to increase in the number of immature erythrocytes. Further studies [21,22] also demonstrated a baseline higher HbA1c in patients and fall after treatment but the reason speculated was different from the one given by Brooks et al. and Sluiter et al. In these studies, the probable explanation of elevated HbAlc in iron deficiency-anaemia at baseline is that, if serum glucose is accepted to remain constant, a decrease in the hemoglobin concentration might lead to an increase in the glycated fraction but the exact mechanism still remains elusive. Nitin Sinha et al. [20] showed drastically different results with values of HbA1c decreasing with fall in haemoglobin values and with treatment these values increased in the next 2 months. The probable reason for these observations given was that the population in study was generally from a lower socio economic strata, being quite poor.So in them, cause of iron deficiency is not just bleeding, malabsorption but nutritional deficiency may be playing an important role in the etiology of iron deficiency. These factors (or other unknown variables) may have a bearing on such results.
Among the 50 patients studied, 33 were female, suggesting that iron deficiency anaemia is more common in women. As expected, the mean hemoglobin and mean serum ferritin levels increased in anaemia patients over 2 months of iron treatment. None of our patients had non responsive iron deficiency anaemia. Our observation of increased HbAlc levels at baseline and its subsequent fall on iron supplementation was in accordance with most of the studies done in the past. There are a number of variable explanations available to explain these findings. We used accepted methodology (ionexchange chromatography) in estimating HbA1c and the analysis was validated in our laboratory. A strict quality control was ensured and samples were tested in assorted manner i.e. the controls and the tests were not analyzed in separate batches but mixed batches were the rule during the period of study.
Iron deficiency anaemia has a straight forward correlation with HbA1c levels and the relationship is inverse between them. This signifies that as the level of haemoglobin drops with increasing severity of iron deficiency in anaemic subjects, at the same time HbA1c levels increase correspondingly. Moreover, with correction of iron deficiency in the anaemic subjects, the HbA1c levels decline to near normal values. This concludes that whenever HbA1c is calculated to detect past three months glycemic status, factors other than glucose also play a part in its calculated value. These other factors should always be kept in mind before ending up with a therapeutic treatment modification. Iron deficiency anaemia being extremely common in Indian settings should always be ruled out when high HbA1c levels are detected and should be corrected on priority to achieve true levels of HbA1c. India being the diabetic capital of the world and HbA1c being such a common investigation in day to day medical practice, should always be interpreted carefully keeping in mind all the factors affecting its value including some very common ones like iron deficiency anaemia. The reason behind this correlation between iron deficiency anaemia and HbA1c is still not clear and various theories exist to explain this. However more large scale studies are required to find out the proper mechanism underlying this correlation.
17206
References
- Telen MJ, Kaufman RE (2004) The mature erythrocyte. In: Greer JP, Forester J (Eds). Wintrobe's clinical hematology. (11thedn). Lippincot: Williams and Wilkins 230.
- (2007) American Diabetes Association. Position statement: Standards of medical care in diabetes-2007. Diabetes Care 30: 1-9.
- Horton BF, Huisman TH (1965) Studies on the heterogeneity of hemoglobin. VII. Minor hemoglobin components in haematological diseases. Br J Haematol 11: 296-304.
- Eberentz-Lhomme C, Ducrocq R, Intrator S, Elion J, Nunez E, et al. (1984) Haemoglobinopathies: a pitfall in the assessment of glycosylated haemoglobin by ion-exchange chromatography. Diabetologia 27: 596-598.
- Bernstein RE (1980) Glycosylated hemoglobins: hematologic considerations determine which assay for glycohemoglobin is advisable. ClinChem 26: 174-175.
- Starkman HS, Wacks M, Soeldner JS, Kim A (1983) Effect of acute blood loss on glycosylated hemoglobin determinations in normal subjects. Diabetes Care 6: 291-294.
- Lind T, Cheyne GA (1979) Effect of normal pregnancy upon the glycosylated haemoglobins. Br J ObstetGynaecol 86: 210-213.
- Hanson U, Hagenfeldt L, Hagenfeldt K (1983) Glycosylated hemoglobins in normal pregnancy: sequential changes and relation to birth weight. Obstet Gynecol 62: 741-744.
- Phelps RL, Honig GR, Green D, Metzger B, Frederiksen MC, et al. (1983) Biphasic changes in hemoglobin A1c concentrations during normal human pregnancy. Am J ObstetGynecol 147: 651-653.
- de Boer MJ, Miedema K, Casparie AF (1980) Glycosylated haemoglobin in renal failure. Diabetologia 18: 437-440.
- Flckiger R, Harmon W, Meier W, Loo S, Gabbay KH (1981) Hemoglobincarbamylation in uremia. N Engl J Med 304: 823-827.
- Paisey R, Banks R, Holton R, Young K, Hopton M, et al. (1986) Glycosylated haemoglobin in uraemia. Diabet Med 3: 445-448.
- Shendurnikaref N (Ed). Iron deficiency is preventable. [Updated on Apr 2007].
- https://www.indiaparenting.com/raisingchild/data/raisingchild063.shtml.
- Brooks AP, Metcalfe J, Day JL, Edwards MS (1980) Iron deficiency and glycosylated haemoglobin A. Lancet 2: 141.
- Sluiter WJ, van Essen LH, Reitsma WD, Doorenbos H (1980) Glycosylated haemoglobin and iron deficiency. Lancet 2: 531-532.
- Mitchell TR, Anderson D, Shepperd J (1980) Iron deficiency, haemochromatosis, and glycosylated haemoglobin. Lancet 2: 747.
- vanHeyningen C, Dalton RG (1985) Glycosylated haemoglobin in iron-deficiency anaemia. Lancet 1: 874.
- Gram-Hansen P, Eriksen J, Mourits-Andersen T, Olesen L (1990) Glycosylated haemoglobin (HbA1c) in iron- and vitamin B12 deficiency. J Intern Med 227: 13336.
- Rai KB, Pattabiraman TN (1986) Glycosylated haemoglobin levels in iron deficiency anaemia. Indian J Med Res 83: 234-236.
- Sinha N, Mishra TK, Singh T, Gupta N (2012) Effect of iron deficiency anaemia on haemoglobin A1c levels. Ann Lab Med 32: 17-22
- El-Agouza I, Abu Shohla A, Sirdah M (2002) The effect of iron deficiency anemia on the levels of hemoglobin subtypes: possible consequences for clinical diagnosis. Clin Lab Hematol 24: 285-289.
- Coban E, Ozdogan M, Timuragaoglu A (2004) Effect of iron deficiency anemia on the levels of hemoglobinAlc in nondiabetic patients. ActaHaematol 112: 126-128.









