Keywords
Microencapsulation, solvent evaporation, plasticizers, glipizide
Introduction
Numerous microspheres preparation methods and materials, which can be incorporated in microspheres, enable precise optimization of sustained release in different physiological conditions. Due to microscopic size, microspheres can be used alone or incorporated in other drug delivery systems and are suitable for various routes of application. Microencapsulation by the solvent evaporation method is a complex process, which can be influenced by many process parameters, e.g. solvent evaporation rate [1], temperature [2, 3], solubility of polymer, drug and excipients in both emulsion phases [4,5], dispersion stirring rate [6], viscosity, solubility, volume and volume ratio between the inner and outer phases [7], the quantity of polymer and drug [8] and the physico-chemical properties and concentration of the stabilizers. In this present study, glipizide microspheres were prepared by solvent evaporation technique using Eudragit RS 100 as a matrix polymer. Liquid paraffin and acetone system were used for the preparation of microspheres. Magnesium stearate was used as a droplet stabilizer to prevent droplet coalescence in the oil medium and n-hexane was added as a nonsolvent to the processing medium to solidify the microspheres. The effect of plasticizers with different concentrations on the percent yield, entrapment efficiency, mean particle size and the drug release of microspheres was investigated.
Materials and Methods
Materials
Eudragit RS 100 was obtained as gift sample from Rohm Pharma, GmbH, Darmstadt, Germany. Glipizide was obtained as gift sample from Dr. Reddy’s Laboratory Ltd., Hyderabad (India). All other chemicals used of analytical grade were purchased from Loba Chemicals Pvt. Ltd., Mumbai (India).
Methods
Preparation of eudragit microspheres
Glipizide loaded eudragit microspheres were prepared by solvent evaporation method and the composition along with formulation code were as given in Table no. 1. The desired amount of eudragit was dissolved in 8.5 ml acetone with stirring to form polymer solution. Required amount of glipizide and plasticizers, dibutyl phthalate (DBT) and diethyl phthalate (DET) were dissolved in polymer solution and mixed thoroughly. To the above solution magnesium stearate (100 mg) was added and stirring continued for 15 minutes. The resultant dispersion was poured into 250 ml beaker containing 100 ml liquid paraffin under stirring. Stirring was continued at 400 rpm for 6 hours until all the acetone evaporated. Finally microspheres were filtered using whatman no. 1 filter paper. The residue was washed with n hexane for 4 to 5 times and dried at room temperature for 24 hrs.
| Formulation Code |
Drug : E-RS100 |
% w/v of
Plasticizers |
| DBT |
DET |
| F1 |
1 : 4 |
-- |
-- |
| F2 |
1 : 4 |
-- |
10 |
| F3 |
1 : 4 |
-- |
20 |
| F4 |
1 : 4 |
10 |
-- |
| F5 |
1 : 4 |
20 |
-- |
Table 1 :Formulation codes with quantities. Drug: Gilipizide, E-RS100: Eudragit RS 100, DBT: Dibutyl phthalate, DET: Diethyl phthalate.
Evaluation of microspheres
Percent yield and entrapment efficiency
Prepared microspheres were weighed after drying, and percent yield was calculated using following formula
Percentage Yield = (Actual weight x 100)/ Theoretical Weight (1)
Microspheres of known weights were stopper tightly in a flask containing 50 ml of 6.8 pH phosphate buffer. The flasks were shaken using orbital shaker for 48 hours to break the beads completely. After 48 hours the solution was filtered using whatman’s No. 1 filter paper and the filtrate was centrifuged using a tabletop centrifuge to remove the polymeric debris. Then the polymeric debris was washed twice with fresh solvent (water) to extract any adhered drug.
The clear supernatant solution was then analyzed for glipizide content by a UV spectrophotometer (JASCO-V500, Japan) at the λ max value of 275 nm. The complete extraction of drug was confirmed by repeating the extraction process on the already extracted polymeric debris. The % entrapment efficiency of the matrix was then calculated as
% Entrapment efficiency = (Drug loading / Theoretical drug loading) x 100. (2)
Micrometric properties
Diameters of the dried microspheres were measured by optical microscopy.
Bulk density and tap density was determined according to following method:
A 50 ml glass cylinder was weighed and filled with 30 ml of sample and reweighed. The opening was secured with parafilm. The cylinder was gently reversed once and the powder was carefully leveled without compacting. Bulk volume was determined after one mechanical tap on a tap density tester (DolphinTM). Tap volume was measured after 2000 taps. Each analysis was repeated twice [9]. Values of bulk density and tap density used to calculate Carrs index [10]. The flow behavior of glipizide loaded eudragit microspheres were determined by Angle of repose. Fixed funnel method was used for determination of angle of repose [11].
Scanning Electron Microscopy (SEM)
Microspheres were coated with a thin gold-palladium layer by sputter coater unit (VG- Microtech, United Kingdom), and the surface topography was analyzed with a Cambridge Stereoscan S120 scanning electron microscope (SEM; Cambridge, United Kingdom) operated at an acceleration voltage of 10 kV.
Fourier transforms Infrared spectroscopy (FTIR)
Fourier transforms Infrared spectroscopy of glipizide and formulation F1, F3 and F5 were recorded using Jasco V5300 (Jasco, Japan) FT-IR system using potassium bromide (KBr) pellet method. Each spectrum was derived from single average scans collected in the region 4000 to 600 cm-1.
In vitro dissolution study
Preparation of calibration curve: Different concentrations of glipizide (2 to 20 μg/ml) were prepared in 6.8 pH phosphate buffer and absorbance is measured by UV- visible spectrophotometer (JASCO-V500, Japan) at the λ max value of 275 nm. Calibration curve was plotted to determine R2 and the equation of straight line which is used to calculate drug release. The dissolution studies were performed by using USP 26 type II dissolution test apparatus (Electrolab TDT-06P, Mumbai, India). Dissolution medium used were phosphate buffer (pH 6.8), each 900 mL, temperature was maintained at 37 ± 2°C and 100 rpm stirring was provided for each dissolution study. Glipizide-loaded eudragit microspheres equivalent to 100 mg of pure drug were used for each dissolution study. Samples were collected periodically and replaced with a fresh dissolution medium. After filtration through Whatman no. 1 filter paper, concentration of glipizide was determined spectrophotometrically at 275 nm. Data obtained from in vitro release studies were fitted to various kinetic equations to find out the mechanism of drug release from beads. The kinetic models used were zero order, second order, Higuchi and Hixon-Crowell model etc..
For zero order release kinetics equation is, Q = K0t, Where, Q = amount of drug release per unit surface area, K0 = Zero order release rate constant and t = time. For first order release kinetics equation is, ln q0/q = -K1 t, Where, q = Amount of drug released per unit surface area, K1= First order release rate constant, q0 = Initial amount, Cs = Saturation Solubility and Ct = Concentration and t = time. For Hixon Crowell release kinetics equation is, W0 1/3 - W1/3 = KHC t, Where, W0 = Initial weight of the particles, W = Weight of the particles, KHC = Hixon Crowell release rate constant and t = time.
For Higuchi release kinetics equation is, Q = KHG t0.5, Where, Q = Amount of drug released per unit surface area of the dosage form, D = Diffusion
co-efficient of the drug, ε = Porosity of the matrix, ζ = tortuosity of the matrix, Cs = Saturation solubility of the drug in the surrounding liquid, KHG = Higuchi release rate constant, A = Conc. of the drug in the matrix, and t = time.
Results and Discussion
Preparation of eudragit microspheres
The Eudragit coated glipizide microspheres prepared by solvent evaporation method. During optimization of the process various parameters were tried. It was found that for less than 1:4 ratio of drug: eudragit concentration respectively there were no proper formation of microspheres. For more than 1:4 ratio of drug: eudragit concentration respectively it was observed that drug particles were precipitated and aggregated masses of particles were formed. At 1:4 ration of drug: eudragit concentration desired microspheres were formed. For less than 400 rpm speed drug got the tendency to settle down on the bottom and at more than 400 rpm speed high turbulence was observed with adhesion of particles to the surface of container. The preparations were stirred for 6 hour gave microspheres with proper strength.
Evaluation of microspheres
Percent yield and entrapment efficiency
The percentage yield and percentage entrapment efficiency of eudragit coated glipizide microspheres were as given in Table 2. It was observed that there was no significant difference in the percent yield for all formulations. Entrapment efficiency for formulation F3 (90 %) was maximum and minimum for formulation F1 (72 %). It was observed that use of plasticizers increased entrapment efficiency of glipizide. For formulations with DBT as plasticizer Entrapment efficiency was comparatively more than with EDT. Again as concentration of plasticizers increases percentage entrapment efficiency of glipizide increases. It may be because with increase in plasticizer concentration increases crosslinking which leads to increases in drug holding capacity.
| FormulationCode |
Yield (%)(n = 3) |
EE (%)(n = 3) |
MPD (µm)(n = 100) |
Carr’s Index (%)(n = 3) |
AOR (°)(n = 3) |
| F1 |
77.7 ± 2.8 |
72.3 ± 1.8 |
710 ± 2 |
13 ± 1 |
22 ± 1 |
| F2 |
78.3 ± 1.6 |
82.9 ± 2.2 |
711 ± 1 |
12 ± 2 |
23 ± 2 |
| F3 |
75.8 ± 1.9 |
90 ± 1 |
709 ± 2 |
12 ± 1 |
22 ± 1 |
| F4 |
73.6 ± 2.1 |
78.4 ± 1.6 |
710 ± 1 |
13 ± 1 |
24 ± 1 |
| F5 |
78.1 ± 2.5 |
80.7 ± 1.8 |
709 ± 1 |
11 ± 2 |
22 ± 1 |
Table 2: Percentage yield and percentage entrapment efficiency (EE), mean particle diameter (MPD), Carr’s index and angle of repose (AOR) of glipizide loaded eudragit microspheres.
Micrometric properties, SEM and FTIR study:
Micromeritic properties of all formulations were as given in table 2. Mean particle diameter (MPD) was in the range of 710 μm. Values of Carr’s index represents its good flowability and compressibility [10]. SEM of microspheres was as given in figure 1. It showed that the microspheres were whitish with spherical in shape. The surface is smooth and showed cross linking by physical observation. It has indicated that increase in concentration of Eudragit RS 100 and plasticizers has increased the encapsulation efficiency with satisfactory yield and also improved the micromeritics properties may be due to spherical shape of the microspheres. FTIR spectra of glipizide and formulation F1, F3 and F5 exhibited identical IR spectra as shown in figure 2 which has indicated that microspheres were not associated with changes at the molecular level. It revealed that no any chemical transition or interaction has occurred during formulation.
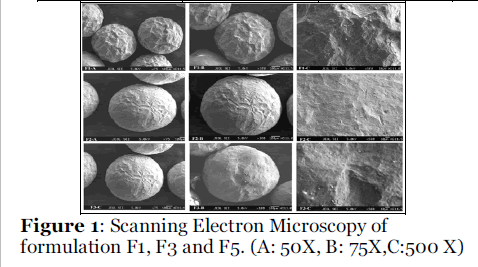
Figure 1: Scanning Electron Microscopy of formulation F1, F3 and F5. (A: 50X, B: 75X,C:500 X).
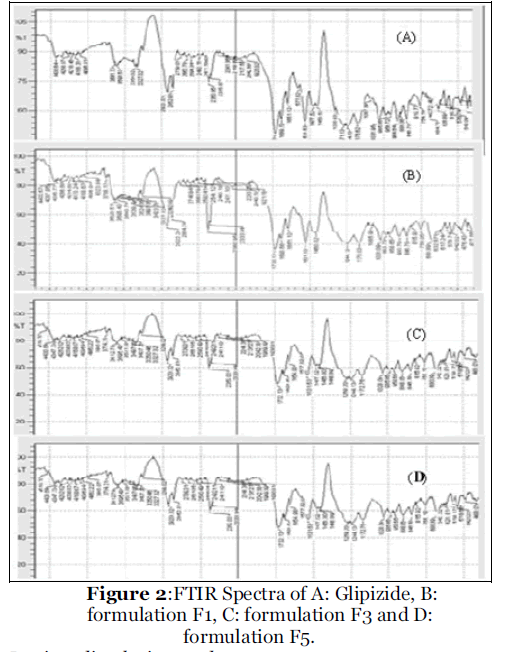
Figure 2: FTIR Spectra of A: Glipizide, B: formulation F1, C: formulation F3 and D: formulation F5.
In vitro dissolution study
The calibration curve prepared using standard solution gives R2 value 0.999, the linearity was observed in the range of 2 to 20 μg/ml and the equation of the line is y = 0.055 X + 0.003. The percentage drug release for all formulations was as given in figure 3. For formulation F1 (plasticizer was not used) 65.9 % drug was release up to 8 hrs. For formulations with plasticizers drug release was sustained. No significant difference was observed in the percentage drug release for formulations with DBT and DET as a plasticizer. Again as concentration of plastizers increases drug release decreases. Model fitting data for dissolution profile was as given in table no. 3. It showed that all the formulations follows Higuchi model [12]. It has indicated that drug release form homogenous matrix was through diffusion. It revealed that increase in concentration of plasticizers decreases the drug diffusion from microsphere might attribute to increase in gel strength with increasing concentration of the polymers [13].
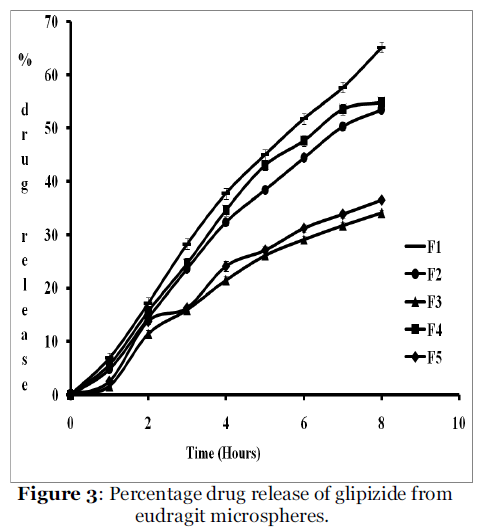
Figure 3: Percentage drug release of glipizide from eudragit microspheres.
| Formulation Code |
R2 values of mathematical
models for dissolution profiles. |
Zero
order |
First
order |
Higuchi |
Hixson-
Crowell |
| F1 |
0.984 |
0.996 |
0.998 |
0.997 |
| F2 |
0.980 |
0.997 |
0.998 |
0.994 |
| F3 |
0.952 |
0.973 |
0.992 |
0.967 |
| F4 |
0.973 |
0.994 |
0.998 |
0.988 |
| F5 |
0.947 |
0.970 |
0.984 |
0.964 |
Table 3: R2 values of mathematical models for dissolution profiles of glipizide from eudragit microspheres.
Conclusion
Glipizide microspheres were successfully prepared by solvent evaporation technique using Eudragit RS 100 as a matrix polymer with dibutyl phthalate and diethyl phthalate as plasticizers. Increase in concentration of plasticizers has increased the percentage entrapment efficiency of glipizide but no significant difference was observed in percentage yield. Microspheres were spherical smooth surface showing cross linking by physical observation. It was also observed that all the formulations follows Higuchi model. It has indicated that drug release form homogenous matrix was through diffusion. It revealed that increase in concentration of plasticizers decreases the drug diffusion from microsphere. Furthermore these microspheres can be orally administered using capsule as a dosage form which will be ideal to maintain sustained release of glipizide.
Acknowledgements
The authors greatly acknowledge Rohm Pharma, GmbH, Darmstadt, Germany and Dr. Reddy’s Laboratory Ltd., Hyderabad, India for providing Eudragit RS 100 and Glipizide as gift samples respectively. The authors are also thankful to Head of the Department, University Department of Pharmaceutical Sciences, Utkal University, Bhubaneswar, Orissa, India for providing research facility.
Conflict of Interest: NIL
Source of Support: NONE
5657
References
- Chung TW, Huang YY, Liu YZ. Effect of the rate of solvent evaporation on the characteristics of drug loaded PLLA and PDLLA microspheres. Int J Pharm 2001; 212: 161-169.
- Bogataj M, Mrhar A, Kristl A, Kozjek F. Eudragit microspheres containing bacampicillin: preparation by solvent removal method. J Microencapsulation 1991; 8: 401-406.
- Wang J, Schwendeman SP. Mechanism of solvent evaporation encapsulation processes: prediction of solvent evaporation rate. J Pharm Sci 1999; 88: 1090-1099.
- Gabor F, Ertl B, Wirth M, Mallinger R. Ketoprofen poly (D, L-Lactic-coglycolic acid) microspheres: influencing of manufacturing parameters and type of polymer on the release characteristics. J Microencapsulation 1999; 16: 1-12.
- Ly J, Wu WY. Bimodal release of theophylline from seed-matrix beads made of acrylic polymers. Pharm Dev Tech 1999; 4: 257-267.
- Mateovic T, Kriznar B, Bogataj M, Mrhar A. The influence of stirring rate on biopharmaceutical properties of eudragit RS microspheres. J Microencapsulation 2002; 19: 29-36.
- Bodmeier R, McGinity JW. Solvent selection in the presence of poly (D, L-Lactide) microspheres prepared by the solvent evaporation method. Int J Pharm 1988; 43: 179-186.
- Lin WJ, Wu TL. Effect of manufacturing conditions on the formation of double walled polymer microspheres. J Microencapsulation 1999; 165: 153-167.
- Zang Y, Law Y, Chakrabarti S. Physical properties and compact analysis of commonly used direct compression binders. AAPS Pharm Sci Tech 2003; 4(4): Article 62.
- Carr R. L. Evaluating flow properties of solids.Chem Engg 1965; 72: 163-168.
- Lachman L, Liberman HA, Konig JL. Theory and Practice of Industrial Pharmacy. P A, Lea and Febiger, Philadelphia, 1986, pp 317.
- Paulo C, Jose M. Modeling and comparision of dissolution profiles. Eur J Pharm Sci 2001; 13: 123- 133.
- Effect of concentration of gellan gum and calcium chloride solution on entrapment efficiency and drug release from calcium gellan beads: Patil SV, Lade PD, Janugade BU, Babar SA, Ghewade YB. Research J Pharm Tech 2009; 2(4): 862-865.









