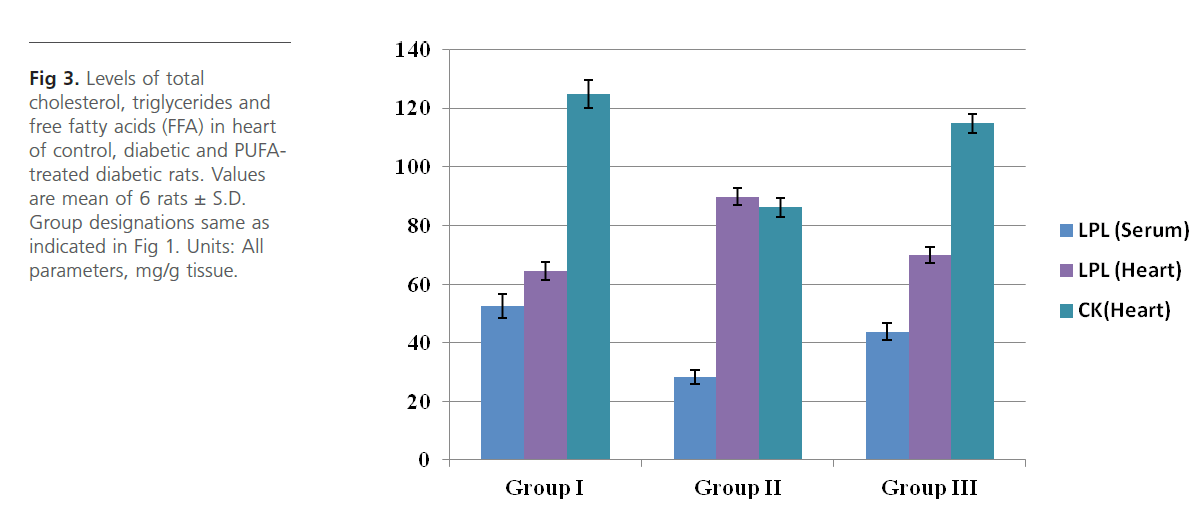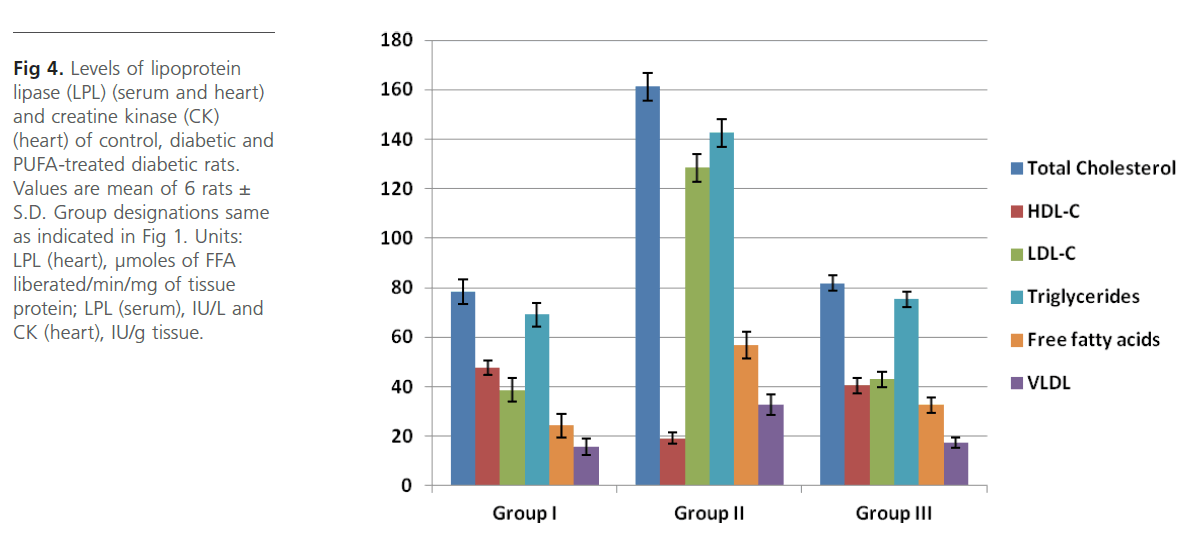Introduction
India tops the list of countries with the highest number of diabetics (Rajeswari et al 2011). The wide prevalence of the disease reveals that it no longer affects only the affluent, though it is most likely to affect those with a sedentary lifestyle and who consume diets that are mainly unhealthy. Diabetes is one of the major causes of premature illness and death in most countries (WHO, 2007). It represents a spectrum of conditions characterized by hyperglyceamia, with irregularities in carbohydrate, lipid and protein metabolism. Diabetes, when not appropriately treated, leads to the development of diabetic complications affecting the heart, kidney, nervous systems and the eyes Years of uncontrolled diabetes would leave a person with diabetic-related complications affecting the heart, kidney, nervous systems and the eyes. Cardiovascular disease, resulting from damage to large blood vessels, causes death of 50% or more of people with diabetes (WHO, 2011). With diabetes, the risk of having heart disease or stroke is doubled . High blood glucose levels over time can lead to increased deposits of fatty materials on the insides of the blood vessel walls (Nichols et al 2004). These deposits may affect blood flow, increasing the chance of clogging and hardening of blood vessels (atherosclerosis). Due to the high prevalence of diabetes worldwide and the morbidity of diabetic complications, extensive research is still being performed to develop new antidiabetic agents.
Polyunsaturated fatty acids (PUFA) from marine fatty fish are known to be heart healthy constituents of fish. The unique characteristics of PUFA and their possible role in preventing coronary heart disease have sparked great interest among clinicians, nutritionists and the public. Several studies have described the beneficial effects of PUFA. Polyunsaturated fatty acids of n-3 family play an important role in the prevention of ischemic heart disease (Vedtofte et al 2011) and favorably influence individual components of the metabolic syndrome (Das, 2010). Beneficial effects of polyunsaturated fatty acids are supposed also in chronic inflammatory and autoimmune diseases (Calder, 2008), psychiatric-neurological diseases (Perica and Delas 2011) and malignant tumors (He et al, 2007). This study is an attempt to explore the effect of PUFA on the heart and blood lipid parameters in streptozotocininduced diabetic albino rats. A Type 2 diabetes (T2D) model should ideally portray an identical biochemical blood profile and pathogenesis to the condition in humans. Streptozotocin is an antibiotic derived from Streptomyces achromogenes and structurally is a glucosamine derivative of nitrosourea. It has a relatively longer half-life (15 min), causes sustained hyperglycaemia for longer durations and develops well-characterized diabetic complications with fewer incidences of ketosis as well as mortality (Arora et al 2009).
Chemicals Polyunsaturated fatty acid extract used for the experiment was prepared form fish liver oil in the Biochemistry and Nutrition Division of our Institute. All of the biochemicals were purchased from Sigma Chemical Company Inc., St Louis, MO, USA. All chemicals used were of analytical grade.
Animals Rats studied were 90-day old male Wistar rats weighing about 215±20g, housed individually in polypropylene cages. The present study was implemented according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), New Delhi, India and authorized by the Animal Ethics Committee of Institute. Diet.
Experimental Design Eighteen rats divided into three groups of six animals each, were used to investigate the hypo-lipidemic lipidaemic effect of PUFA extract in experimental diabetes. All animals were held in standard cages at an animal facility in our institute at constant room temperature (22°C), under a 12-hour light/dark cycle. Water was given ad libitum and animals were weighed weekly. The groups were as follows: Group I, non-diabetic control; Group II, diabetic control; Group III, diabetic rats given PUFA extract (100 mg/kg). The PUFA extract was orally administered via an intragastric tube (0.6 ml/rat) on a daily basis for 27 days. Diabetes was induced in experimental groups II and III of rats by intraperitoneal injection of streptozotocin (60 mg/kg of body weight) in 0.1 mol/l citrate buffer (pH 4.5). Non-diabetic control rats received placebo. After the last treatment (day 27), blood was collected with 1% EDTA and plasma was separated for biochemical analyses. Rats were fasted overnight and sacrificed following chloroform anesthesia. The heart was excised, weighed, cut into small pieces of approximately 100 to 200 mg, frozen immediately, and stored at -20°C for analysis. Blood was collected with 1% EDTA and plasma was separated for analysis of lipid parameters. Diabetic status was confirmed in the STZ-treated rats by measuring the fasting plasma glucose (Harisha, 2008) after 72 h. For each group, plasma glucose level was determined at the beginning of the experiment, 72 hours after STZ administration (only for group II and group III) and at the end of the experiment. Rats with plasma glucose levels above 200mg/dl were considered diabetic and used in experiments. Treatments with PUFA extract were started on day 3 after STZ-injection.
Analytical Methods Body weight, water intake and feed intake were monitored during the entire course of study. Heart samples were weighed and homogenized by standard procedures. Lipids were extracted from an aliquot of the heart homogenate according to the procedure of Folch et al, 1957. The total amount was calculated after evaporation of aliquot to constant weight. Plasma and heart total cholesterol [Parekh and Jung (1970)], triglycerides [Rice (1970)], and free fatty acids [Horn and Menahan (1981)] were determined. Plasma lipoproteins, HDL and LDL (Burstein and Scholnick, 1972) and VLDL (Total plasma cholesterol - (HDL+LDL) cholesterol = VLDL cholesterol) were estimated. Plasma and heart tissue lipoprotein lipase (Dole, 1956) and heart tissue creatine kinase were measured (Rosalki, 1967) Protein was determined using the method of Lowry, 1951.
Statistical Analysis Results are expressed as mean ± SD. Each sample was tested in duplicate. One-way analysis of variance (ANOVA) was carried out, and the statistical comparisons among the groups were performed with Tukey’s test using a statistical package program (SPSS 10.0 for Windows). A p value of less than 0.05 was considered significant.
Results
Group 2 diabetic rats showed significant (p<0.01) weight loss, polydypsia, polyphagia, hyperglyceamia when compared to Group 1 normal control rats (Figure 1). Group 2 rats also had significantly high (p<0.001) plasma total cholesterol, LDL-C, triglycerides and free fatty acids and low HDL-C and phospholipids (Figure 2). Heart tissue content of lipid fractions in diabetic rats: cholesterol, triglyceride and fatty acids were significantly (p<0.001) high whereas the levels of phospholipid show a decrease in comparison with the control rats (Figure 3). In diabetic rats (group 2) a considerable (p<0.001) increase in the activity of lipoprotein lipase the enzyme responsible for the turnover of lipoproteins and supply of free fatty acids for energy production, in heart tissue was observed, when compared to the non-diabetic control rats (Figure 4). Concomitantly there was a significant (p<0.001) lowering of the activity of the enzyme in plasma of diabetic rats when compared to Group 1 control rats (Figure 4). The activity of creatine kinase, the heart specific enzyme, was significantly (p<0.001) low in both serum and heart tissue of Group 2 diabetic rats when compared to the normal control rats (Figure 4).
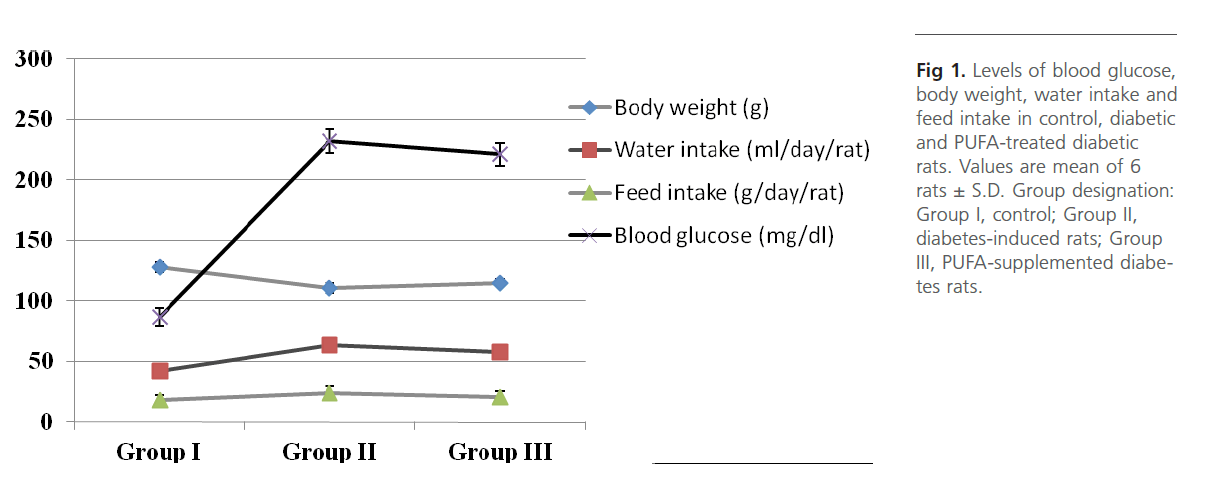
Figure 1: Levels of blood glucose, body weight, water intake and feed intake in control, diabetic and PUFA-treated diabetic rats. Values are mean of 6 rats ± S.D. Group designation: Group I, control; Group II, diabetes-induced rats; Group III, PUFA-supplemented diabetes rats.
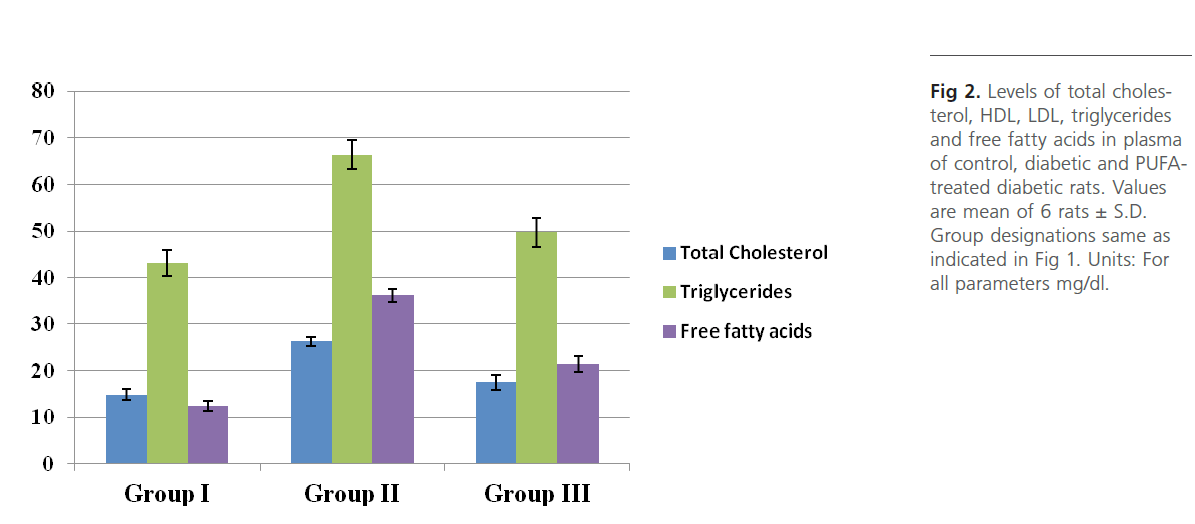
Figure 2: Levels of total cholesterol, HDL, LDL, triglycerides and free fatty acids in plasma of control, diabetic and PUFAtreated diabetic rats. Values are mean of 6 rats ± S.D. Group designations same as indicated in Figure 1. Units: For all parameters mg/dl.
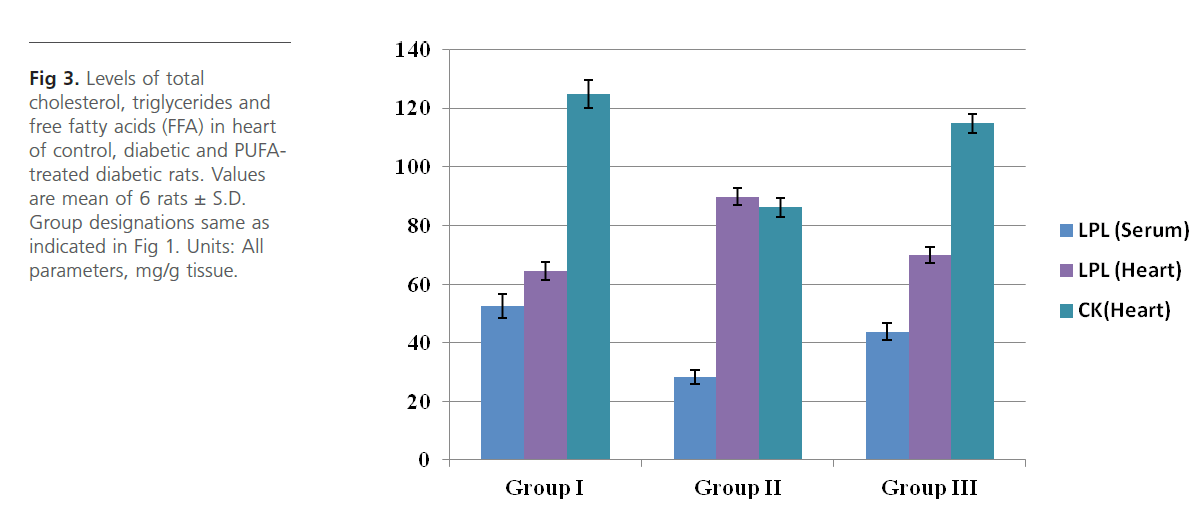
Figure 3: Levels of total cholesterol, triglycerides and free fatty acids (FFA) in heart of control, diabetic and PUFAtreated diabetic rats. Values are mean of 6 rats ± S.D. Group designations same as indicated in Figure 1. Units: All parameters, mg/g tissue.
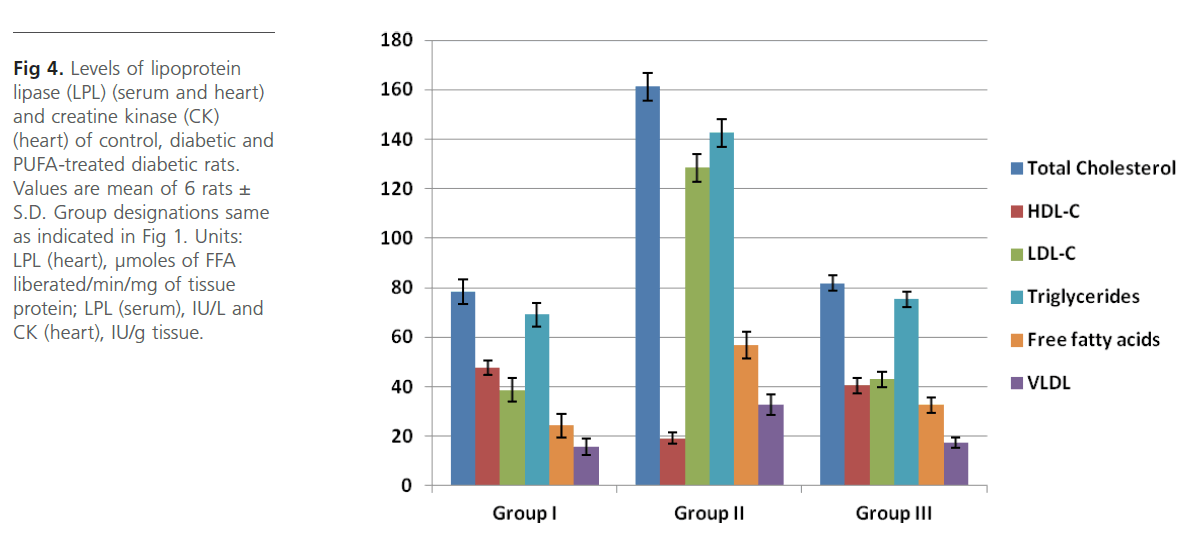
Figure 4: Levels of lipoprotein lipase (LPL) (serum and heart) and creatine kinase (CK) (heart) of control, diabetic and PUFA-treated diabetic rats. Values are mean of 6 rats± S.D. Group designations same as indicated in Figure 1. Units: LPL (heart), μmoles of FFA liberated/min/mg of tissue protein; LPL (serum), IU/L and CK (heart), IU/g tissue.
Intra-gastric administration of PUFA extract in Group 3 rats has caused significant changes in the content of various biochemical parameters studied during the course of this study. First, PUFA-fed rats showed small yet significant (p<0.05) weight gain in comparison to Group 2 diabetic rats but did not show any significant change in polyphagia, polydypsia and hyperglyceamia that were seen in Group 2 diabetic rats (Figure 1). The levels of total cholesterol, LDL-C, triglycerides and free fatty acids were significantly (p<0.001) lowered and the content of phospholipids and HDL-C were significantly (p<0.001) elevated in the plasma of PUFA-administered rats when compared to diabetic rats (Figure 2). The content of cholesterol, triglycerides and fatty acids were significantly (p<0.001) lowered in heart tissue of PUFA-administered rats than in diabetic rats (Figure 3). A significant (p<0.001) decrease was observed in the activity of the LPL enzyme of heart, whereas a considerable (p<0.001) increase was noted in the enzyme’s activity in the plasma of PUFA-supplemented diabetic rats when compared to diabetic rats (graph). In the PUFA-fed rats the activity of creatine kinase in serum and heart tissue was significantly (p<0.001) enhanced when compared to diabetic rats (Figure 4).
Discussion
Diabetes mellitus is a disorder of carbohydrate, lipid and protein metabolism that affects many organs. This study describes the role of LPL, the status of CK and the manner in which PUFA administration affects the alterations in lipid content that occur in induced diabetes. In the present study it is shown that PUFA (1) protects against the diabetes-induced variations in various lipid parameters, (2) restores plasma LPL activity, (3) reduces heart LPL activity and (4) preserves heart CK activity. The high levels of total cholesterol, LDL cholesterol triglyceride and free fatty acids and low HDL-C seen in plasma of Group 2 diabetic rats may be attributed to the low activity of LPL in plasma which may be responsible for the lowered rate of lipoprotein metabolism and clearance from blood. Sorenson et al (2010) report that diabetic ketotic patients had low plasma LPL activity and HDL-C, high plasma triglycerides and cholesterol and that plasma triglyceride and cholesterol were inversely related to lipoprotein lipase activity. Hypertriglyceridemia that was observed in Group 2 diabetic rats in the present study correlates with the fact that this is a common feature in patients with insulin resistance, diabetes and obesity, and is positively correlated to deleterious tissue lipid deposition (Subramanian & Chait, 2011). Diminished systemic LPL activity is suggested to be one mechanism resulting in impaired clearance of circulating lipoproteins especially triglyceride-rich VLDL, and ensuing hypertriglyceridemia [Gonzales & Orlando, 2007].
As we have seen in the study, there was a significant rise in the content of various lipid fractions like cholesterol, triglycerides and free fatty acids in the heart tissue of Group 2 diabetic rats as well. In a study by Jia-hong Xue it was shown that high glucose promotes intracellular lipid accumulation in vascular smooth muscle cells that goes on to develop into atherosclerotic plaques. Phospholipid content in heart of diabetes-induced decreased significantly and is in agreement with the results of other workers. In a study of effect of selenium and vitamin E on lipids, peroxides, and fatty acid distribution in experimental diabetes, Douillet et al 1998 reported that phospholipid levels decreased in heart and liver of diabetic rats.
PUFAs are fatty acids with two or more double bonds and are generally 18-20 carbons in length. The PUFA of marine origin are usually rich in oleic, linolenic, arachidonic, eicosapentaenoic (EPA), docosahexaenoic acids (DHA). In the present study supplementation of PUFA in Group 3 rats did not alter the state of glycaemia but significantly reduced the levels of circulating lipids and lipoproteins, viz. total cholesterol and LDL-C, triglycerides and free fatty acids and augmented the content of HDL-C in Group 3 diabetic rats. PUFA supplemented Group 3 diabetic rats also had reduced content of lipid fractions in heart tissue and showed elevated levels of phospholipid. Group 3 rats have shown a significant increase in the activity of plasma LPL activity which may be responsible for the lowering of various lipid fractions in plasma as well as heart tissue. In a study on transgenic rabbits that have global over expression of LPL, attenuation of hyper-triglyceridemia and reduced deposition of body fat were observed (Koike et al 2004) which implies that systemic increases in LPL could have beneficial effects on whole body tissue lipid metabolism. PUFA supplementation may have helped in lowering the circulating levels of LDL and VLDL triglycerides by one or more of the following mechanisms, viz., a reduction in the absorption of dietary fatty acids, thereby reducing VLDL formation in the gut, enhancing plasma lipoprotein lipase activity and a reduction in the hepatic VLDL synthesis and secretion.
The high activity of LPL observed in heart of Group 2 diabetic rats in the present study may be to compensate for the reduced amount of glucose available as fuel for energy production due to insulin deficiency (Rodrigues et al 1997). The LPL acts on lipoproteins LDL and VLDL secreted by liver and gut respectively to release free fatty acids that serve as the alternate fuel for the heart. However, the dramatic increase in free fatty acids influx as seen in this study is not without negative consequences. In the heart, elevated free fatty acids and subsequent TG synthesis have been implicated in a number of metabolic, morphological, and mechanical changes, and also in lipotoxicity (Pulinilkunnil, & Rodrigues, 2006). It was described that during lipotoxicity, free fatty acids accumulated and either by themselves or via production of second messengers such as ceramides, provoked cell death (Wei, Y. et al., 2009). Given the pivotal function of LPL in regulating the levels of lipid and lipoprotein fractions in plasma and heart and also in free fatty acids delivery, and free fatty acids’ contribution in mediating cellular lipotoxicity, examining the regulation of this enzyme is crucial for understanding the metabolic basis of diabetic heart disease. PUFA have the ability of modulating LPL activity as demonstrated by Saxena and Goldberg, 1989. Using fatty acid/bovine serum albumin (BSA) solutions, it was shown that the release of LPL from endothelial cell surfaces using polyunsaturated and saturated fatty acids was 60% and 28%, respectively. The proposed mechanism for this effect is a fatty acid feedback system in which the accumulation of fatty acids obstructs lipoprotein lipase hydrolysis thereby regulating its activity
The reduced activity of creatine kinase in heart of Group 2 diabetic rats observed in the present study is in agreement with studies reported earlier. Mitani et al 2000 showed that the expression of mRNAs of creatine kinase (CK)-B and CK-M and myocardial CK-MB activity are suppressed in diabetic rats. In addition to contractile abnormalities in diabetes, this disease causes disturbances in the function of cardiac subcellular organelles, including the mitochondria and is in particular associated with several abnormalities in energy metabolism. In Group 3 diabetic rats, PUFA administration has restored the activity of heart creatine kinase. These results agree with a study reported by Ovide-Bordeaux et al 2004. Through DHA (PUFA) feeding experiments in diabetic rats, it was revealed that established that DHA modified the fatty acid composition of cardiac membranes, including mitochondria and increased mitochondrial creatine kinase (mi-CK) activity.
Conclusion
In general, favourable effects of polyunsaturated fatty acids can be explained by their ability of influencing of cellular metabolic functions, incorporation into membrane phospholipids, modulation of enzymes and signal molecules as well as by direct impact on gene expression. Polyunsaturated fatty acids are said to have high therapeutic potential, which results from the combined action on different levels of cell functions. For instance according to Zak et al 2005, in conditions like hyper- and dyslipoproteinemias and fatal myocardial infarction, their effect is nearly pharmacological. Polyunsaturated fatty acids (PUFA), from marine source, contain a large proportion of n-3 and n-6 long chain fatty acids which by way of getting incorporated in myocardial membranes may have a positive influence on cardiovascular performance. As a result of PUFA’s conclusive impact on cardiac lipid parameters and heart specific enzymes like creatine kinase and lipoprotein lipase, it may be recommended as a dietary supplement for patients with diabetic heart disease.
Acknowledgements
The authors thank the Director, CIFT for granting permission to publish this paper. The technical assistance provided by technical personnel of B&N Division of CIFT is gratefully acknowledged
1933
References
- Amanda M Gonzales and Robert A Orlando 2007. Role of adipocytederived lipoprotein lipase in adipocyte hypertrophy. Nutrition & Metabolism , 4:22
- Burstein M, Scholnick HR and Morfin R. 1970. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J. Lipid Res. 11: 853–895.
- Calder PC. 2008. Session 3: Joint Nutrition Society and Irish Nutrition and Dietetic Institute Symposium on ‘Nutrition and autoimmune disease’ PUFA, inflammatory processes and rheumatoid arthritis. Proc Nutr Soc., 67(4):409-18.
- Christina M. Sorensen, Jie Ding, Qibin Zhang, Thierry Alquier, Rui Zhao, Patricia W. Mueller, Richard D. Smith, Thomas O. Metz, Perturbations in the lipid profile of individuals with newly diagnosed type 1 diabetes mellitus: Lipidomics analysis of a Diabetes Antibody Standardization Program sample subset, Clinical Biochemistry, Volume 43, Issue 12, August 2010, Pages 948-956,
- CK Somjen, D., Shen, M., Stern, N. and Mirsky, N. (2006), Diabetes modulates differentially creatine kinase-specific activity responsiveness to estradiol-17β and to raloxifene in rat organs. Journal of Cellular Biochemistry, 99: 133–139.
- Das, U.N. Metabolic Syndrome Pathophysiology: The Role of Essential Fatty Acids, 2010.John Wiley & Sons 284 pages
- Dole, V. P. 1956. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J . Clin. Invest. 35: 150-1 54.
- Excoffon KJ, Liu G, Miao L, Wilson JE, McManus BM, Semenkovich CF, et al. Correction of hypertriglyceridemia and impaired fat tolerance in lipoprotein lipase-deficient mice by adenovirus-mediated expression of human lipoprotein lipase. Arterioscler Thromb Vasc Biol 1997;17:2532 – 9.
- Folch J,Lees M and Solan-Stanely G H., 1957. A simple method for the isolation and purification of total lipids from animal tissue. J.Biol. Chem.: 226;497-509
- Gregory A. Nichols, Christina M. Gullion, Carol E. Koro, Sara A. Ephross, and Jonathan B. Brown
- Harisha, S., 2008. Biotechnology Procedures and Experiments Handbook, Laxmi Publications Pvt Ltd., 694 pages
- He, Q., Shkarin, P., Hooley, R. J., Lannin, D. R., Weinreb, J. C. and Bossuyt, V. I. J. (2007), In vivo MR spectroscopic imaging of polyunsaturated fatty acids (PUFA) in healthy and cancerous breast tissues by selective multiple-quantum coherence transfer (Sel-MQC): A preliminary study. Magnetic Resonance in Medicine, 58: 1079-1085.
- Horn W T and Menahan L A., 1981. A sensitive method for the determination of free fatty acids in plasma. Journal of Lipid Research;22: 377–381.
- K. Rajeswari , V. Vaithiyanathan, T. Gurumoorthy. Modeling Effective Diagnosis of Risk Complications in Type 2 Diabetes – A Predictive model for Indian Situation. European Journal of Scientific Research 54: (2011), pp.147-158.
- Koike T, Liang J, Wang X, Ichikawa T, Shiomi M, Liu G, et al. Overexpression of lipoprotein lipase in transgenic Watanabe heritable hyperlipidemic rabbits improves hyperlipidemia and obesity. J Biol Chem 2004;279:7521 – 9
- Lowry O H., Rosebrough N J, Farr A L and Randall R J., 1951.Protein measurement with Folin-phenol reagent. J.Biol.Chem; 193:265-275.
- Marina Mandelsamen Perica and Ivancica Delaš, 2011. Essential Fatty Acids and Psychiatric Disorders Nutr Clin Pract 26: 409-425
- Matthias Spindler, Kurt W Saupe, Rong Tian, Saadia Ahmed, Mohammed A Matlib, Joanne S Ingwall, Altered Creatine Kinase Enzyme Kinetics in Diabetic Cardiomyopathy. A31P NMR Magnetization Transfer Study of the Intact Beating Rat Heart, Journal of Molecular and Cellular Cardiology, Volume 31, Issue 12, December 1999, Pages 2175-2189
- Mitani, S., Okumura Kenji, Matsui Hideo, Toki Yukio, Hashimoto Hidekazu, Ito Takayuki, Hayakawa Tetsuo, 2000. Insulin alters cardiac muscle creatine kinase activity. Heart and Vessels, 15: 23-29
- Ovide-Bordeaux S, Grynberg A. Docosahexaenoic acid affects insulin deficiency- and insulin resistance-induced alterations in cardiac mitochondria. Am J Physiol Regul Integr Comp Physiol, 2004; 286: R519-R527
- Parekh A C and Jung D H., 1970. Cholesterol determination with ferric acetate uranylacetate and sulphuric acid –ferrous sulphate reagents. Anal. Chem.; 42: 1423-1427.
- Rice CL. 2000. Muscle Function at the Motor Unit Level: Consequences of Aging. Topics in Geriatric Rehabilitation; 15: 70-82
- Rodrigues B, Cam MC, Jian K, Lim F, Sambandam N, Shepherd G. 1997. Differential effects of streptozotocin-induced diabetes on cardiac lipoprotein lipase activity. Diabetes;46:1346 –53.
- Rosalki, S. B., An improved procedure for serum creatine phosphokinase determination. J. Lab. Clin. Med. 69,696 (1967).
- Sachin Arora, Shreesh Kumar Ojha and Divya Vohora, 2009. Characterisation of Streptozotocin Induced Diabetes Mellitus in Swiss Albino Mice. Global Journal of Pharmacology, 3 (2): 81-84
- Savitha Subramanian, Alan Chait, Hypertriglyceridemia secondary to obesity and diabetes, Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids, (In Press, Corrected Proof) Available online 8 October 2011, ISSN 1388-1981,10.1016/j.bbalip.2011.10.003. (https:// www.sciencedirect.com/science/article/pii/S1388198111001946)
- The Incidence of Congestive Heart Failure in Type 2 Diabetes:An update Diabetes Care August 2004 27:1879-1884
- Thomas Pulinilkunnil, Brian Rodrigues, 2006. Cardiac lipoprotein lipase: Metabolic basis for diabetic heart disease. Cardiovascular Research 69: 329 – 340
- Uday Saxena, Ira J. Goldberg, 1990. Interaction of lipoprotein lipase with glycosaminoglycans and apolipoprotein C-II: Effects of freefatty- acids, Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism, 1043:161-168
- Vedtofte, Mia Sadowa, Jakobsen, Marianne U, Lauritzen, Lotte, Heitmann, Berit L. 2011. Dietary α-linolenic acid, linoleic acid, and n–3 long-chain PUFA and risk of ischemic heart disease .The American Journal of Clinical Nutrition doi: 10.3945/ ajcn.111.018762
- Wei, Y. et al., 2009. Yuren Wei, Dong Wang, Christopher L Gentile, Michael J Pagliassotti, 2009. Reduced endoplasmic reticulum luminal calcium links saturated fatty acid-mediated endoplasmic reticulum stress and cell death in liver cells. Molecular and Cellular Biochemistry, 331(1-2), p.31-40.
- Zák A, Tvrzická E, Zeman M, Vecka M. 2005. Pathophysiology of and clinical significance of polyunsaturated fatty acids n-3 family. Cas Lek Cesk. ;144 Suppl 1:6-18.