Abstract
Background: This is the first case report of a stepped novel anti-inflammatory treatment plan for a complicated, markedly disabling neuropsychiatric condition with multiple comorbidities. The treatment consisted of 1) hyperbaric oxygen therapy, 2) combination therapy of infused ketamine applied concurrent with transcranial magnetic stimulation, 3) administration of perispinal etanercept, and finally 4) non-invasive transnasal Sphenopalatine Ganglion block.
Methods and findings: The patient was a 34-year-old Caucasian unemployed female who presented with lifelong symptoms of a regulatory disorder of childhood, epilepsy, reflex sympathetic dystrophy, and multiple concussions that ultimately led to marked disability in activities of daily living. Following the sequential treatment, the patient showed marked clinical improvement and progressed from major incapacitation to an independent life, resuming school and taking a parttime job. Indeed, successive improvements occurred after hyperbaric oxygen therapy in the realms of distress tolerance, resilience, and social interaction and then continued after downward titration of oral medications (which required the use of the ketamine/transcranial magnetic stimulation treatment), and further improved after administration of perispinal etanercept. Finally, a Sphenopalatine Ganglion block provided migraine relief. Single-photon emission computer tomography imaging was done before the beginning of treatment, two months later (after hyperbaric oxygen therapy), and at the conclusion of the perispinal etanercept injections. Results from both follow-up SPECT scans validated the clinical impression by showing substantial and additive blood flow increases in previously deficient brain areas.
Conclusions: We provide evidence of successful treatment of a disabling neuropsychiatric condition via the sequential application of a novel anti-inflammatory treatment plan including four procedures, all known for their anti-inflammatory effects. The use of functional imaging provided a useful biomarker for the efficacy of this sequential treatment.
Keywords
Neuropsychiatric disorder; Hyperbaric oxygen therapy; Ketamine; Transcranial magnetic stimulation; Perispinal etanercept; Brain SPECT; Functional brain imaging; Misophonia
Abbreviations
Atmospheres Absolute (ATA); Computed Tomography (CT); Electroencephalogram (EEG); Generalized Anxiety Disorder (GAD); Hyperbaric Oxygen Therapy (HBOT); Magnetic Resonance Imaging (MRI); Perispinal Etanercept (PSE); Reflex Sympathetic Dystrophy (RSD); Single Photon Emission Computed Tomography (SPECT); Sphenopalatine Ganglion (SPG); Transcranial Magnetic Stimulation (TMS); Tumor Necrosis Factor (TNF); repetitive Transcranial Magnetic Stimulation (rTMS)
Introduction
The treatment and rehabilitation of patients with a history of multiple neuropsychiatric conditions, aggravated by repeated mild traumatic brain injuries and ultimately leading to marked functional disability, is known to be challenging [1,2]. In particular, pre-existing neuropsychiatric problems can become treatment resistant or intractable when the effects of multiple traumatic brain injuries are superimposed. All of these conditions have been shown to induce variable levels of neuroinflammation [3-6].
We present the very favorable results obtained over a period of over three years, consequent to the administration of some lesser-used methods of anti-inflammatory treatment, in a patient who had previously been intractably ill. The treatments included 1) hyperbaric oxygen therapy (HBOT), 2) combination therapy of infused ketamine concurrent with transcranial magnetic stimulation (TMS), 3) injections of perispinal etanercept (PSE), and 4) non-invasive trans-nasal Sphenopalatine Ganglion (SPG) block. HBOT is believed to have anti-inflammatory effects, reducing the excess of proinflammatory cytokine activation such as TNF-alpha and facilitating improvement by provocation of stem cell activity [7-10]. The combination therapy of infused ketamine administered concurrent to TMS appears to address the abnormalities in brain circuits found both in treatment resistant depression and treatment resistant central pain [11-16]. Additionally, ketamine is known to act as a TNF-alpha inhibitor, thus favoring an anti-inflammatory effect [17]. PSE injections induce tumor necrosis factor (TNF)-alpha modulation directly to the central nervous system and act to normalize the inflammatory response in stroke, traumatic brain injury, and encephalopathic conditions [18-20]. The SPG is known to decrease migraine intensity [21] and also to have an antiinflammatory effect [22]. We thus aimed to provide information about the use of novel anti-inflammatory procedures, in a step-wise approach, for the treatment of a patient with disabling, treatment resistant, neuropsychiatric comorbidities.
Methods
A 35-year-old Caucasian unemployed woman presented to our clinic with life-long symptoms of a regulatory disorder of childhood, including dysregulated head pain (of migraineous intensity), stomach pain, parasomnia, panic attacks, and emotional dysregulation. Despite these difficulties, the patient had been able to complete college. Up until age 24, she had not held steady employment, but was actively involved in supporting her church and in other volunteer work.
When the patient was 24 years old, she impacted a tree with her car after having a seizure while driving on the highway. This resulted in a concussion as well as fractures to both of her ankles. Subsequent to that event, the patient was diagnosed by her psychiatrist (not at the present clinic) with generalized anxiety disorder (GAD), secondary insomnia, adjustment disorder with mixed anxious and depressed mood, migraine headaches, and partial epilepsy with impairment of consciousness and progressive worsening of lower extremity pain. The patient was treated with medication and counseling, and continued her part-time work and involvement in churchrelated activities, while continuing to live with her parents.
At 31 years old, while traveling overseas, the patient was subject to another concussion, without loss of consciousness but resulting in a large ecchymosis upon her right forehead. This occurred after a minivan in which the patient had been riding rolled over and she was projected from the lateral bench on one side to the other side of the enclosed vehicle. Upon return home, she consulted with a neurologist for pain, fear, and dull cognition. A computed tomography (CT) scan performed a month later was normal, prompting the neurologist to conclude that any symptoms the patient experienced as a result of the trauma would subside. However, both her neuropsychiatric and pain symptoms worsened, eventually becoming the most severe she had ever experienced. In addition to her previous conditions, the patient began having unusual auditory and visual experiences. She would hear familiar voices giving her advice, see an aura around a person, or see nearby objects as though they were far away from her. Nonetheless, she was not psychotic, meaning she maintained her insight that these perceptual symptoms were unusual. She also developed extreme misophonia and photophobia, and required constant use of noise suppression headphones along with dark sunglasses during her waking hours. The patient described herself as spending the next three years of her life isolated and “completely in the dark,” incapable of participating in almost any activity. She was unable to leave her house due to misophonia, head pain of migraineous intensity and lower extremity pain. Indeed, she seldom left her room. Her parents cared for her throughout this time. Her depression worsened substantially, including feelings of severe loneliness.
By the time the patient arrived at our clinic approximately four years after the last accident, she was practically unable to engage in usual daily activities let alone in social events and employment. Her medication history included over 40 types, including antidepressants, anticonvulsants, antianxiety agents, antipsychotics, stimulants, a mood stabilizer (lithium), and pain medication (including Methadone). Her extended-family history was positive for ambulatory depression and high-functioning alcoholism. Her own substance use history was not significant (except for pain medication). Thyroid tests performed by our clinic were normal. Consequently, even though the patient had a history of borderline thyroid levels, hypothalamic hypothyroidism post-concussion was deemed unlikely. Other hormone levels were unremarkable, with the exception of low cortisol. We made the clinical diagnosis of multiple comorbidities including depression, reflex sympathetic dystrophy (RSD), misophonia photophobia, post-concussion syndrome, and auditory and visual perceptual distortions.
Results
A brain SPECT functional imaging with 99m-Tc HMPAO was performed at baseline in our clinic. This is a non-invasive nuclear medicine procedure that can detect functional changes in various gray matter locations. It does so by mapping the blood flow distribution in both cortical and subcortical gray matter areas of the brain. The results are displayed in a two-dimension (2D) and three-dimension (3D) color rendition of relative perfusion maps as well as in a black and white volumetric rendition thresholded at the 65% level [23-26]. Results from this assessment revealed extensive bilateral hypoperfusion most accentuated in the frontal and temporal lobes (see the upper row of Figure 1). Such bilateral findings on brain SPECT have been seen in various conditions including head trauma without localized impact, where the injuries were caused by a combination of acceleration/ deceleration and rotational forces, even in the absence of magnetic resonance imaging (MRI) findings [27,28]. They have also been seen in long standing depression and various neurotoxic exposures, including iatrogenic ones. A 3 Tesla MRI scan performed shortly after showed normal appearance except for a new 9x6 mm enhancing structure located cephalad from the left internal auditory canal. A special MRI for the auditory canal was performed three weeks later and suggested that the aforementioned signal on the left was likely due to inflammatory changes.
Based on clinical evaluation and on the results of the baseline SPECT, our first line of intervention was to refer the patient for HBOT. She followed a standard protocol consisting of 60-minute treatments administered five days per week over a one-month period at a pressure of 1.5 atmospheres absolute (ATA). She received a total of 40 treatments, which resulted in continuous but slow improvement in the realms of distress tolerance, resilience, and social interaction, including increased ability to leave her home. A second SPECT done a month after the conclusion of HBOT showed improved perfusion in practically every area of the cortex and in some subcortical structures (see the second row and green arrows in Figure 1).
At this point we decided to initiate a medication washout, the main goal being progressive discontinuation of anticonvulsant medications and opioid analgesics. In order to facilitate the opioid washout and improve the patient’s chronic pain, a combined TMS/ketamine treatment was started next. It consisted of 21 sessions over a six-month period, as described in our previous publications [16,29-32]. Immediately prior to the first combined treatment session, the patient completed two days of rTMS pre-treatment. Each pre-treatment day consisted of four 30-minute sessions of rTMS, with a 45-minute resting interval between each session. One hertz TMS was continuously applied, without off cycles, at 115% of the motor threshold, based on three years of observational data from our clinic. The TMS head coil (manufactured by Neotonus, Inc., Marietta, GA, USA) was placed at the midline anteriorly on the patient’s scalp. This placement was intended to maximize stimulation of the medial prefrontal area that overlays the anterior cingulate region, which has been implicated in depression and central pain syndromes [11,12].
In each session of the combined treatment, the patient first underwent five minutes of TMS. Intravenous ketamine (Ketalar®, manufactured by Par Sterile Products LLC, Parsippany, NJ, USA) was then administered over a 30-minute span, concurrent to and bracketed within a 40-minute TMS treatment (the patient concluded with five minutes of TMS post infusion). This protocol was repeated 21 times over six months at approximately equal intervals. The ketamine dosage was gradually increased from 25 mg delivered over 30 minutes at the first session to 80 mg delivered over the same period by the last. The ketamine dose adjustment, also previously described [16], was based on the three main factors: 1) the patient’s increased tolerance for the experience of infused ketamine, 2) her reduction in symptoms, and 3) a clinical evaluation performed approximately three days after each treatment. The outcome of this treatment was that the patient was able to discontinue most of her medications including the methadone-based analgesia. An electroencephalogram (EEG) performed one year into the washout (once she was free of anticonvulsant medication) was normal.
Improvements started after the first month of the combined TMS/ketamine treatment, and continued to accrue even after the end of the whole series. The patient showed marked improvements in her RSD pain, she was no longer continuously clinically depressed, the unusual auditory and visual experiences subsided, and her energy levels increased. She returned to church activities and started traveling to social functions, for example participating in a wedding in another state. However, she remained emotionally reactive and irritable, and her ability to perform daily activities was still erratic, as she continued to struggle with periodic head pain, anger, and/or episodes of depression, and was cognitively still somehow limited. Therefore, approximately six months after completion of the TMS/ketamine treatment series, another line of intervention was started, namely the PSE injections [18-20,33] (The method of perispinal administration of etanercept was used under license from the patent holder, TACP IP, LLC.).
The patient showed remarkable immediate improvements in cognition, affect, and vocabulary following the first 25 mg PSE injection. Second and third injections of the same dosage were given at approximately one-week intervals to maximize clinical effects.
At the end of the PSE injections, a third SPECT was performed. This scan revealed very significant additional improvements, as shown in the third row of Figure 1.
During the one-year follow-up period after the PSE injections, the patient showed significant improvements in nearly every area of functioning. Her cognition and affect continued to improve, the frequency of her depressive episodes and irritability decreased significantly, and she was able to read, write, and communicate with the alacrity of a graduate student. She was able to regularly engage in daily acts of living and increasingly participated in work, church, and other social activities. Additionally, she regained her driver’s license. Particularly noteworthy was the fact that she was asked and agreed to participate in speaking engagements for traumatic brain injury patient audiences.
Finally, eight sessions of non-invasive trans-nasal SPG block were performed to treat persisting head pain. We used a modified protocol based on Candido et al. [34] by administering Marcaine (bupivacaine) 0.5% solution, 0.3 cc in each nare, weekly over two months. This resulted in substantial alleviation of the migraine pain, although some psychosensory features remained.
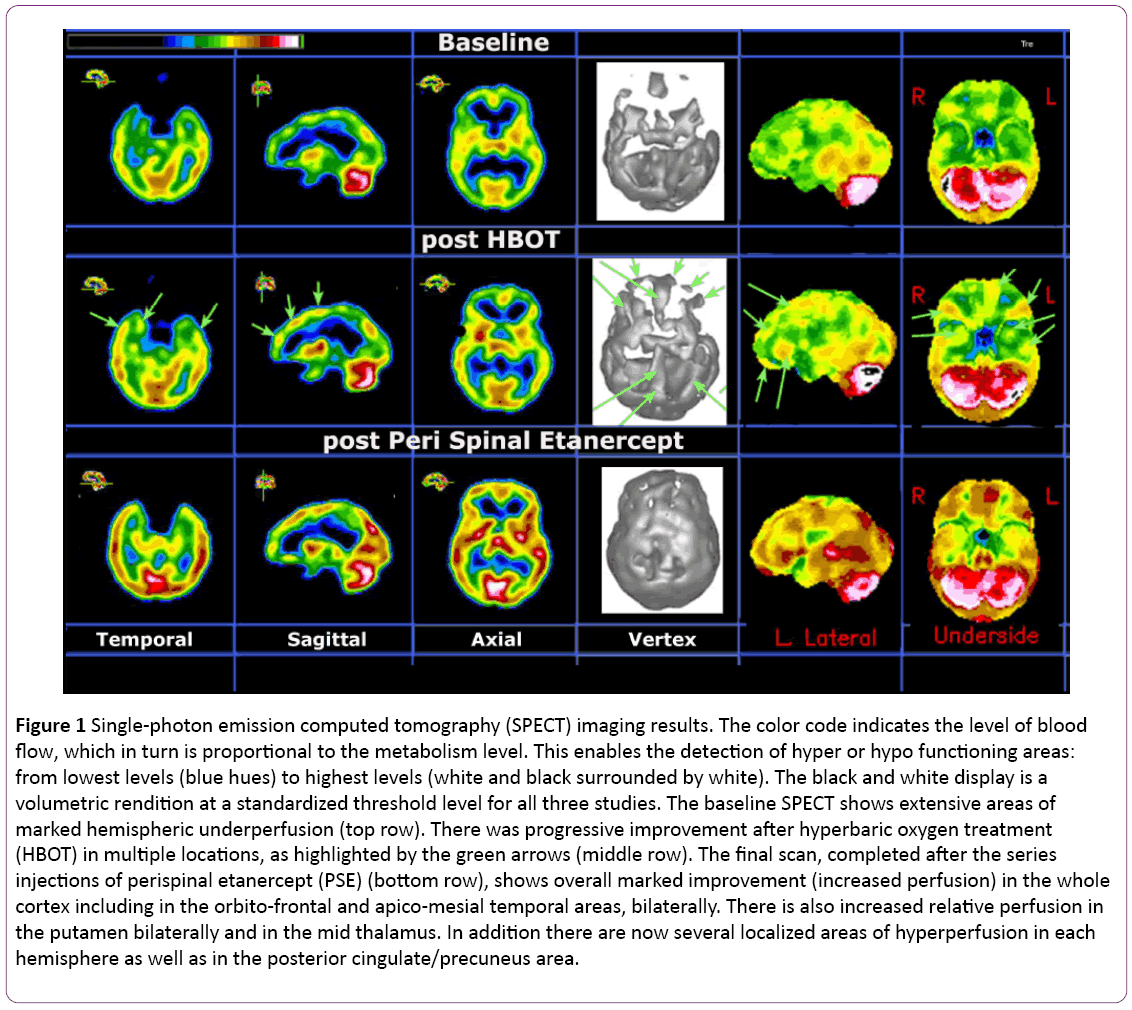
Figure 1: Single-photon emission computed tomography (SPECT) imaging results. The color code indicates the level of blood flow, which in turn is proportional to the metabolism level. This enables the detection of hyper or hypo functioning areas: from lowest levels (blue hues) to highest levels (white and black surrounded by white). The black and white display is a volumetric rendition at a standardized threshold level for all three studies. The baseline SPECT shows extensive areas of marked hemispheric underperfusion (top row). There was progressive improvement after hyperbaric oxygen treatment (HBOT) in multiple locations, as highlighted by the green arrows (middle row). The final scan, completed after the series injections of perispinal etanercept (PSE) (bottom row), shows overall marked improvement (increased perfusion) in the whole cortex including in the orbito-frontal and apico-mesial temporal areas, bilaterally. There is also increased relative perfusion in the putamen bilaterally and in the mid thalamus. In addition there are now several localized areas of hyperperfusion in each hemisphere as well as in the posterior cingulate/precuneus area.
Discussion
The sequence of events in this very difficult case point to the fact that, besides commonly used medication and rehabilitation therapy, there are additional therapeutic means that should be considered in the treatment of complex neuropsychiatric cases, especially when complicated by multiple traumatic brain injuries. The chronic problems our patient presented with since childhood, i.e., regulatory disorder of childhood [35] followed by affective disorder, partial epilepsy, RSD pain, and multiple traumatic brain injuries, are known to have neuro-inflammation in common [3-6]. The use of HBOT, although well documented, is still considered controversial by some. Recent publications have reported variable outcomes in great part because of using protocols that differ significantly in depth, duration, frequency and number of sessions [36-38]. It appears that HBOT can be effective in decreasing chronic inflammation in the brain by reducing pro-inflammatory cytokine activation and through provocation of stem cell activity [7-10]. The repeat brain SPECT indicated the valuable contribution of this technique as a functional imaging biomarker.
The combination therapy of TMS/ketamine infusion has been recently patented and successfully applied in a variety of conditions involving various treatment-resistant neuropsychiatric disorders, including pain and depression [16,29-32,39,40]. In the present case, this brought the patient to a higher functional level in part due to the fact that it enabled a drastic cut in the number and amount of drugs she was taking. Nonetheless, this was still short of adequate independence in, or better tolerance for, daily activities. Therefore, the decision was made to start PSE. This technique works in part by inducing TNF-alpha modulation directly to the central nervous system, thus normalizing the inflammatory response [18-20,33]. The subsequent clinical amelioration and marked improvement on the SPECT functional imaging validated this decision. Finally, the patient’s persisting head pain was addressed by the non-invasive SPG block [21,22,34].
The long follow-up the present study showed an additional benefit of the stepwise anti-inflammatory treatment, namely that it prevented the recurrence of the patient’s main symptoms of depression, RSD pain, cognitive deficits, and allodynic head pain with misophonia.
Conclusion
When faced with complex, chronic neuropsychiatric conditions aggravated by multiple traumatic brain injuries, it may be beneficial to go beyond the more common medication and rehabilitation procedures, adding one or more of the novel forms of anti-inflammatory treatment outlined above. Our findings may be of particular interest given that depression and pain can be inextricably linked, exacerbating each other and thus becoming very difficult to treat, especially in the context of added multiple traumatic brain injuries. This vicious cycle appears to have been successfully interrupted, and recurrence of future symptoms avoided, by the sequence of novel therapeutic interventions. The use of Brain SPECT functional imaging, in this context, provided a useful biomarker for confirmation of the therapeutic benefit of this sequential anti-inflammatory treatment.
Acknowledgements
There are no acknowledgements to be made.
Funding
No external funding sources were involved in any aspect of this case study: its conception or design, the acquisition of data, the analysis and interpretation of data, or the drafting or revising of the manuscript.
Competing and Conflicting Interests
The authors have no competing or conflicting interests to declare.
11216
References
- Whelan GR (2010) Predictors of psychiatric disorders following traumatic brain injury. Journal of Head Trauma Rehabilitation 25: 320-329.
- Mayer CL, Huber BR, Peskind E (2013) Traumatic brain injury, neuroinflammation, and post-traumatic headaches. Headache 53: 1523-1530.
- Rathbone ATL (2015) A review of the neuro- and systemic inflammatory responses in post-concussion symptoms: Introduction of the "post inflammatory brain syndrome" PIBS. Brain, Behavior, and Immunity 46: 1-16.
- Mitchell RHB, Goldstein BI (2014) Inflammation in children and adolescents with neuropsychiatric disorders: A systematic review. Journal of the American Academy of Child & Adolescent Psychiatry 53: 274-296.
- Najjar S (2013) Neuroinflammation and psychiatric illness. Journal of Neuroinflammation 10: 1-24.
- Serafini G, Rihmer Z, Amore M (2015) The role of glutamate excitotoxicity and neuroinflammation in depression and suicidal behavior: Focus on microglia cells. Neuroimmunal Neuroinflmmation 2: 127-130.
- Vlodavsky E, Palzur E, Soustiel JF (2006) Hyperbaric oxygen therapy reduces neuroinflammation and expression of matrix metalloproteinase-9 in the rat model fo traumatic brain injury. Neuropathology and Applied Neurobiology 32: 40-50.
- Milovanova TN (2009) Hyperbaric oxygen stimulates vasculogenic stem cell growth and differentiation in vivo. Jounral of Applied Physiology 106: 711-728.
- Heyboer MI (2014) CD34+/CD45-dim stem cell mobilization by hyperbaric oxygen-Changes with oxygen dosage. Stem Cell Research 12:638-645.
- Li F (2011) Hyperbaric oxygenation therapy alleviates chronic constrictive injury-induced neuropathic pain and reduces tumor necrosis factor alpha production. Anesthesia & Analgesia 113: 626-633.
- Mayberg HS (2005) Deep brain stimulation for treatment-resistant depression. Neuron 45: 651-660.
- Kanda M (2003) Transcranial magnetic stimulation (TMS) of the sensoriomotor cortex and medial frontal cortex modifies human pain perception. Clinical Neurophysiology 114: 860-866.
- Thut G (2011) Rhythmic TMS causes local entrainment of natural oscillatory signatures. Current Biology 21: 1176-1185.
- Fuggetta G, Noh NA (2013) A neurophysiological insight into the potential link between transcranial magnetic stimulation, thalamocortical dysrhythmia and neuropsychiatric disorders. Experimental Neurology 245: 87-95.
- Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, et al. Optogenetic stimulation of infralimbic PFC reproduces ketamine's rapid and sustained antidepressant actions. Proceedings of the National Academy of Sciences of the United States of America, in press.
- Best SRD (2014) Rapid relief of treatment resistant depression by facilitated ketamine infusion: A preliminary report. Activitas Nervosa Superior 56: 28-36.
- De Kock M, Loix S, Lavand'homme P (2013) Ketamine and peripheral inflammation. CNS Neuroscience & Therapeutics 19: 403-410.
- Clark IA, Vissel B (2015) A neurologist's guide to TNF biology, and to the principles behind the therapeutic removal of excess TNF in disease Neural Plasticity 1-10.
- Tobinick E (2010) Perispinal etanercept: A new therapeutic paradigm in neurology. Expert Review of Neurotherapeutics 10: 985-1002.
- Tobinick E (2014) Immediate neurological recovery following perispinal etanercept years after brain injury. Clinical Drug Investigations 34: 361-366.
- Leong MS, Gjolaj MP, Gaeta RR (2013) Sphenopalatine ganglion block, in Comprehensive Treatmetn of Chronic Pain by Medical, Interventional, and Integrative Approaches. Springer: New York. 303-307.
- Garutti I (2014) Intravenous lidocaine decreases tumor necrosis factor alpha expression both locally and systemically in pigs undergoing lung resection surgery. Anesthesia & Analgesia 119: 815-828.
- Catafau AM (2001) Brain SPECT in clinical practice. Part I: perfusion. Journal of Nuclear Medicine 42: 259-271.
- Holman BL, Devous MD (1992) Functional brain SPECT: the emergence of a powerful clinical method. Journal of Nuclear Medicine 33: 1888-1904.
- Minoshima S (1994) Anatomical standardization: linear scaling and nonlinear warping of functional brain images. Journal of Nuclear Medicine 35: 1528-1537.
- Juni JE (2009) Procedure guidelines for brain perfusion PSECT using 99mTc radiopharmaceuticals 3.0. Journal of Nuclear Medicine Technology 37: 191-195.
- Pavel DG (2006) Viewing the functional consequences of traumatic brain injury by using brain SPECT. Brain and Cognition 60: 211-213.
- Raji CA (2014) Clinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: A systematic review. PLOS ONE 9: e91088.
- Best SRD (2014) Combined ketamine/transcranial magnetic stimulation treatment of severe depression in bipolar I disorder. Journal of ECT 30: e50-e51.
- Best SRD (2015) Combined ketamine and transcranial magnetic stimulation for treatment resistant depression in the context of chronic OCD: A case report. Neuropsychiatric Electrophysiology 1:1-4.
- Best SRD, Griffin BP (2014) Combination therapy utilizing ketamine and transcranial magnetic stimulation for treatment-resistant depression: A case report. International Journal of Neuroscience 1-3.
- Best SRD, Griffin BP, Pavel DG (2015) Ketamine and transcranial magnetic stimulation treatment for bipolar II disorder: A case report. Journal of Medical Case Reports 9: 1-4.
- Ignatowski TA (2014) Perispinal etanercept for post-stroke neurological and cognitive dysfunction: Scientific rationale and current evidence. CNS Drugs 28: 679-697.
- Candido KD (2013) A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain Physician 16: E769-E778.
- Dale LP (2011) Infant regulatory disorders: Temperamental, physiological, and behavioral features. Journal of Developmental Behavioral Pediatrics 32: 216-224.
- Boussi GR (2013) Hyperbaric oxygen therapy can improve post-concussion syndrome years after mild traumatic brain injury - randomized prospective trial. PLoS ONE 8: e79995.
- Harch PG (2013) Hyperbaric oxygen therapy for post-concussion syndrome: Contradictory conclusions from a study mischaracterized as sham-controlled. Journal of Neurotrauma 30: 1995-1999.
- Efrati S (2015) Hyperbaric oxygen therapy can diminish fibromyalgia syndrome-prospective clinical trial. PLoS ONE 10: e0127012.
- Fasick V (2015) The hippocampus and TNF: Common links between chronic pain and depression. Neuroscience & Biobehavioral Reviews 53: 139-159.
- Spengler RN, Ignatowski TA (2016) Disruption of brain-TNF homeostasis elicits maladaptive alterations producing chronic pain. BrainImmune Trends.






