Keywords
|
| Restraint stress; Prenatal; Maternal; Neurobehavioral; Development; Rat |
Introduction
|
| Recent studies have raised the attention that the origin of future health disturbances may lie in fetal development and early pre- and postnatal environment. During intrauterine life the developing body is extremely susceptible to environmental adversities since it undergoes dynamic changes [1,2]. Such adversities affecting the pregnant mother can result from malnutrition, exogenous glucocorticoids, and maternal emotional disturbances resulting from natural or man-made disasters as well as interpersonal tensions, anxiety, depression, daily challenges at home or workplace [3,4]. A typical response in these situations is the elevation of stress hormone, cortisol and the activation of hypothalamic-pituitary-adrenal axis (HPAaxis). While we know that cortisol is transported through the placenta and it has a critical role in tissue and organ maturation, the exact mechanism how its excess level may cause alterations in fetal development has not been completely understood [2,5]. In normal pregnancy the level of cortisol in fetus is about 10-fold lower than in the mother because it is converted into inactive cortisone by placental 11β-hydroxysteroid-dehydrogenase-2 (11β-HSD2) enzyme. Animal experiments suggest that maternal stress may lead to decreased functioning of 11β-HSD2 enzyme resulting in high amount of glucocorticoids affecting the developing brain [3,5-7]. They might have detrimental effects on cell proliferation, differentiation and synapse formation, mainly in amygdala, hippocampus and limbic cortical areas leading to short- and long-term postnatal consequences [8-12]. Barros et al. observed a long-lasting astroglial reaction, a reduced dendritic arborization and loss of synapses in the brain of adult offspring suffering intrauterine stress during the last week of pregnancy [13]. It is common in human prenatal stress literature that preterm delivery, reduced birth weight and infant temperament problems such as irritability, sleeping and feeding difficulties are associated with maternal anxiety/stress. Nimby and later Hansen and co-workers reported strong relationship between structural malformations and adverse life event during pregnancy [14,15]. Reports have also shown delayed motor development, impaired cognitive functioning and behavioral, psychopathologic disorders during childhood and later in adulthood [16-19]. |
| Whereas in human studies the genetic and social factors cannot be excluded, with the help of animal models the timing and length of stress can be controlled. A widely used animal model of prenatal stress is restraining the pregnant rat in a transparent cylinder. The aforementioned human findings correlate with studies where the adult offspring of rat dams exposed to restraint stress during pregnancy presented depressive-like behavior, spatial memory attention, cognitive disturbances and impaired coping with repeated stressful situations [7,20,21]. |
| Most studies focus on the effects of prenatal stress in adulthood. Relatively little is known about the early neurodevelopmental consequences of such experiences and their predictive value. Following the maturation of certain neural reflexes and motor coordination may give us an insight into the neurodevelopment of prenatally stressed animals. It has been shown that reflex ontogeny in rodents is influenced by several factors such as undernutrition, maternal care, toxic agents, environmental enrichment, genetic background [22-30]. We have previously described that perinatal hypoxic and toxic injuries remarkably delay the neurobehavioral development but 3-hr-long maternal separation induces only slight changes [31-34]. Ten et al. reported for the first time that short-term neuro-functional performance correlate with long-term functional deficits and suggested the predictive value of early evaluation [35]. Thus we aimed to assess the early neurobehavioral responses, observing possible gender differences, of offspring whose mothers suffered restraint stress during early or late periods of pregnancy. |
Materials and Methods
|
|
Animals
|
| Animal housing, care and application of experimental procedures were in accordance with institutional guidelines under approved protocols (No: BA02/2000-15024/2011, University of Pecs following the European Community Council directive). |
| Three-month-old nulliparous female Wistar rats were mated with male rats. Pregnancy was determined by daily vaginal smear examination. The day at which the smear was sperm positive was considered as gestational day 0 (GD0). Pregnant rats were housed under standard conditions ad libitum food and water access in a temperature controlled room on 12 hr light/dark cycle. |
|
Restraint stress
|
| Animals were subjected to prenatal restraint stress by placing them into a transparent plastic cylinder for 60 minutes twice a day for seven days during the light cycle. The schedule of sessions was not fixed to reduce a possible habituation to restraint stress. Dams were divided into three groups according to the timing of prenatal restraint stress (PRS) procedure: early or mid-pregnancy stress (MPS) from GD7 to GD14; late-pregnancy stress (LPS) from GD14 to GD21 and control dams were left undisturbed during the whole pregnancy. |
|
Neurobehavioral testing
|
| After birth, offspring (n of control = 11; n of MPS = 21; n of LPS = 18) were examined daily from postnatal day (PD) 1 to PD 21 for the maturation of neural signs and reflexes. Possible gender differences were also evaluated. Tests were based on previous descriptions [31,32,34]. Weight was measured daily until 3 weeks of age. Day of appearance of physical signs such as eye opening, incisor eruption, ear unfolding was recorded. The following neural signs and reflexes were examined daily by an examiner blinded to the groups: 1) Surface righting reflex: pups were placed in supine position and the time in seconds to turn over to prone position and place all four paws onto the surface was recorded. 2) Negative geotaxis: animals were placed head down on an inclined grid of 30 cm height. Hind limbs of the pups were placed in the middle of the grid. The day they began to turn around and climb up with their forelimbs reaching the upper rim was recorded. The test was considered negative when the animals did not complete it within 30s. From the day of positive test, the time in seconds to reach the top rim was also recorded. 3) Crossed extensor reflex: the left hind paw was pinched with forceps and the animal was observed for the extension of the right leg. The day of disappearance of the reflex, when it was replaced by a more complex response, was noted. 4) Sensory reflexes: ear and eyelid were gently touched with a cotton swab and the first days of ear twitch reflex and of the contraction of the eyelid were registered. 5) Limb placing: fore- and hind paws were faced with their back side to a table edge and the first day when the animal placed the paw on the top of table was recorded. 6) Limp grasp: fore- and hind limbs were touched with a thin rod, and the first day of grasping onto the rod was recorded. 7) Gait: animals were placed in the center of a white paper circle of 13 cm in diameter, and the day they began to move off the circle with both forelimbs was recorded. 8) Auditory startle: the first day of the startling response to a clapping sound was observed. 9) Air righting: animals were dropped head down onto a bed of shavings from a height of 50 cm, and the first day of landing on four feet was recorded. |
|
Statistical analysis
|
| Results of neurobehavioral signs and reflexes of offspring from the observed groups were analyzed by using one-way or two-way ANOVA respectively, with Dunnett’s multiple comparison tests after Bartlett’s test for equal variances (GraphPad Prism 6.0. software, CA USA). Results are shown in mean ± SEM, the difference is considered to be significant when p < 0.05. |
Results
|
|
Somatic development
|
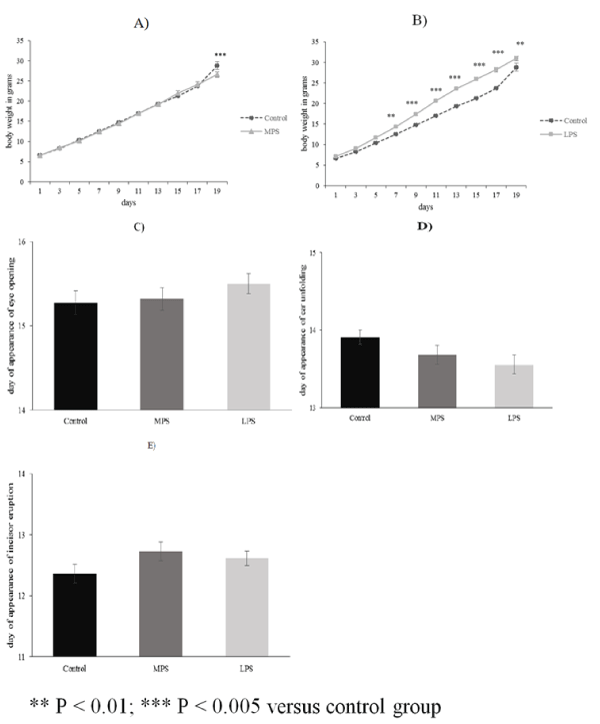
| The average body weight of pups from LPS groups (n = 18) was significantly higher from PD7 than in the control group (n = 11) (Figure 1B). Weight gain of MPS pups (n = 22) did not differ from that of the control subjects except on PD19 when it was lower than the average body weight of the controls (Figure 1A). The days of eye-opening, incisor eruption and ear unfolding were not significantly different in any of the examined groups (Figures 1C-1E). No gender differences were observed in the examined groups regarding body weight and somatic development. |
|
Neurological reflexes
|
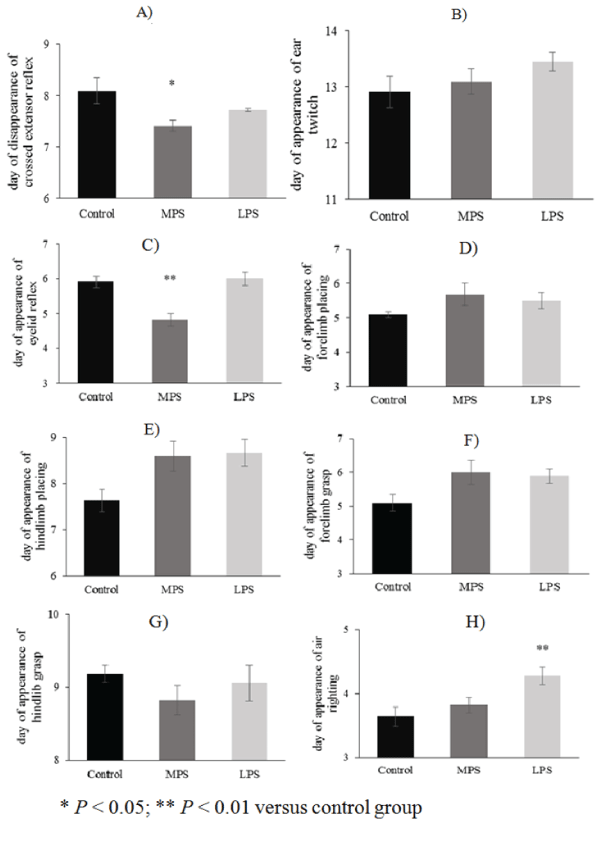
| The daily performance of reflexes in the LPS group improved parallel with that of the control pups, except for auditory startle and air righting, which appeared earlier (13.16 ± 0.14 vs. 13.90 ± 0.09; p = 0.011; F(2;48) = 4.92; MS = 0.47; SS =22.7) or delayed (4.27 ± 0.13 vs. 3.63 ± 0.15; p = 0.0085 ; F(2;48) = 5.28; MS = 0.32; SS = 15.4), respectively (Figures 2H-2I). Some neurological reflexes, such as crossed extensor (7.409 ± 0.107 vs. 8.09 ± 0.25; p = 0.0375; F(2;48) = 3.52; MS = 0.49; SS = 23.8), eyelid reflex (4.81 ± 0.18 vs. 5.9 ± 0.16; p<0.0001; F(2;48) = 13.1; MS = 0.62; SS = 30.2) and auditory startle (13.18 ± 0.18 vs. 13.9 ± 0.09; p = 0.029; F(2;48) = 4.92; MS = 0.47; SS = 22.7), developed faster in MPS rats than in controls (Figures 2A, 2C, 2I), but the appearance of other reflexes did not differ from the controls (Figures 2B, 2D-2H). |
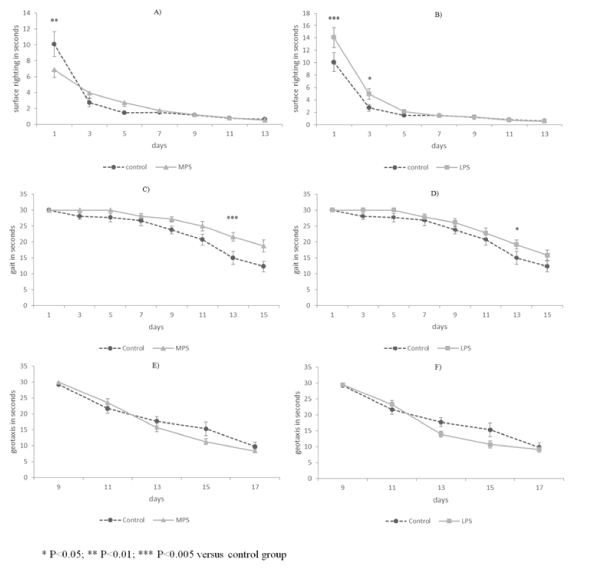
| Surface righting reflex was performed significantly more slowly by both stressed groups only on the first day (Figures 3A-3B). Negative geotaxis was executed similarly in all groups (Figures 3E-3F). Animals affected by early intrauterine stress performed gait reflex more slowly from PD11 (Figure 3C). It was performed more slowly only on PD13 by LPS rats, while at other time points no differences were observed between control and late-intrauterine stressed animals (Figure 3D). |
Discussion
|
| In the present study we described the early neurobehavioral development of pups exposed to prenatal stress in different periods of pregnancy. The observed alterations after a 60- minutes-long restraint stress twice a day were not so remarkable in any of the groups. A subtle faster development was observed in the appearance of eyelid and auditory startle reflexes, and in the disappearance of crossed extensor reflex in the pups subjected to prenatal stress during the middle of pregnancy. Pups exposed to stress in the last week of intrauterine life displayed a delay in air righting and showed a slight enhancement in the appearance of auditory startle. |
| In rodents, the first two weeks of age is the critical period of neuronal maturation and pups reach adult level of basic neuronal reflex development at the end of the third postnatal week [22,23,36,37]. Using the same battery of tests, we are able to make comparative analysis based on experiments carried out in the last 10 years [38]. We found that neonatal hypoxia and asphyxia led to extremely severe retardations in our developmental model [31,33]. Rats undergoing perinatal asphyxia or exposure to hypoxic environment after unilateral carotid ligation had marked delays in the appearance of most reflexes and even 4-5 days delay in the appearance of some maturation signs. They also completed behavioral tests in markedly slower time [38]. However, maternal deprivation or excitotoxic injury caused by monosodium glutamate treatment led to only transient delay in the development of physical signs, neurological reflexes and motor coordination. Recently, similar results have been found in neonatal inflammation: maternal exposure to lipopolysaccharide leads to transient motor dysfunction in rats [39]. Our present results reveal that rats exposed to prenatal stress showed only subtle positive or negative differences compared to their unstressed mates. These findings are similar to the result of our previous study where we evaluated the neurodevelopmental outcome of early postnatal stress, namely a 3-h-long maternal deprivation, which did not induce drastic changes either [34]. Interestingly, physical landmarks, such as ear and eye opening, analyzed in a PRS protocol slightly different from this study, showed no difference between PRS offspring and control pups, not even after a cross fostering procedure, suggesting that either prenatal or postnatal interventions do not exert a profound influence on certain initial physical landmarks [40,41]. However, testis descent was delayed and anogenital distance decreased pointing to a hormonal misbalance that was in fact later demonstrated [40,42]. |
| Prenatal stress is known to have long-term consequences at several levels. In the background of the described various behavioral alterations and abnormalities different biochemical and signaling effects have been reported [7,20,21]. Among others, impaired adaptation to stressful conditions, increased vulnerability to toxic and traumatic lesions, memory impairments and delayed psychomotor development have been described [43]. Changes in neurotransmitter levels and metabolism, hormonal alterations and gene expression patterns have been documented [12,42,44,45]. In this respect, it is worth mentioning that many neurobehavioral impairments may be related to changes in the dopamine (DA) neurotransmission. The analysis of certain aspects of the dopaminergic metabolism at early ages (PD 7), such as levels of tyrosine hydroxylase (TH) and of specific transcription factors (TF) revealed that TH was decreased in substantia nigra (SN) and ventral tegmental area (VTA) and that Nurr-1, a specific DA TF, showed an increase in VTA. These results show that prenatal stress exerts an early life impairment of the regulatory enzyme of DA synthesis and changes in key factors of the formation and maintenance of midbrain DA pathways [46]. |
Conclusion
|
| The early changes following a perinatal lesion are thought to have prognostic value. However, based on our present findings the deleterious consequences of prenatal stress are not apparent during the early developmental stages, at least not detectable with the battery of test most widely used to examine neurobehavioral development. These findings also draw the attention of the need of careful awareness in later ages in spite of the normal neurobehavioral development of newborns exposed to prenatal stress. |
Funding
|
| PTE-MTA “Lendulet” Program, Janos Bolyai Research Scholarship of the Hungarian Academy of Sciences, TÁMOP-4.2.2.B-15/KONV-2015-0011, OTKA K104984 and PD109644, Hungarian Brain Research Program – Grant No. KTIA_13_NAP-A-III/5 |
Figures at a glance
|
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
|
| |
|
|








