Keywords
Aba; Abacha; Deterioration; Food values; Fungal components; Aflatoxin
Introduction
Abacha is a staple food which is processed from cassava (Manihot esculenta Crantz) as snack in Aba metropolis at Eastern part of Nigeria. This food is becoming a daily intake for people and it has a bed rock as a cheap and affordable energy source. It is prepared as dry or wet product through the shredding or slicing of boiled or soaking of cassava tubers for 8-24 h followed by washing or drying as may be desired. However, its production still follows conventional procedure of cassava shredding and not on modern procedure which varies and numerous across different communities (Ekwu et al. [1].
It plays a large part of household sustenance in eastern Nigeria to combat hunger, it represents 5% food expenditure (Ekwu et al. 2013) and 20.5% Calories diet daily intake for the local populace [2]. Reported that the nutrient composition of the ‘abacha’ includes moisture content range of 9.53-10.48%, 1.07-1.66% protein, 2.06-2.56% ash, 1.72-1.95 fibre, 0.39- 0.58% fat and 83.59-85.05% carbohydrate on the dried weight basis; while the wet Abacha has a pH of 5.60–5.80, 0.047-0.063 total titratable acidity (TTA), and 7.80-10.41 mg/100 g hydrogen cyanide (HCN). Despite the nutritional values of Abacha, it is still susceptible to microbial contamination during production and storage which can lead to ‘bio-deterioration’ i.e. spoilage and secretion of some toxic substances such as aflatoxins which are of public health importance. Many of the potential contaminating microbes include fungi.
Many groups of fungi mostly filamentous (Deuteromycota) fungi such as Penicillium, Aspergillus, Fusarium, Rhizopus, Trichoderma and Mucor have been successfully isolated from cassava food products they are regarded as ‘bio-deteriorating fungi’ that can cause reductions in food value or nutrients. Stored food materials usually becomes dried with gradual reduction in the water contents stressing the fungi mycelium and triggers production of some fungal toxin called mycotoxins such as aflatoxins. Aflatoxins are the most deadly mycotoxins, they are produced by Aspergillus species and are known to be one of the most deadly carcinogens due to detrimental effects they can exert on their consumers, and this is also confirmed by the International Agency for Research on Cancer [3]. Who classified them as Group 1 carcinogens. There are five different types of aflatoxins that exist in nature; they are aflatoxin B1 (AFB1), aflatoxin B2 (AFB2), aflatoxin G1 (AFG1), aflatoxin G2 (AFG2), and aflatoxin M1 (AFM1), respectively.
Many traditional ways used for food preparation and storage such as heating and roasting can reduce the aflatoxin contents in foods [4]. Ranging values in nutritional contents of sausage were observed by [5] due to different methods adopted for its preparation, another report was that of Lopez-Garcia and Park (1998), Park (2002) and Fandohan et al. [6] who determined the fates of aflatoxin and fumonisin during different traditional way of preparing maize and maize foods. Aspergillus species can grow at temperature range of 10-50°C while others such as Fusarium tricinctum have optimum growth temperature of 25°C but produces T-2 toxin best near freezing temperatures. Penicillium martensii usually produces penicillic acid rapidly at 20-30°C, but considerably more toxin eventually accumulates around 4 to 10°C. Most mycotoxin contamination finds their way into crop during the storage stage. Hence, proper care must be taken into consideration when storing food products that are wholesome and apparently healthy. Pre-storage treatment or handling should take care of certain basic issues. Mycotoxin levels in foods should be monitored using appropriate sampling and testing programs.
However, the fungi compositions and effect of storage methods on nutritional and aflatoxin contents in Abacha to the best of our knowledge have not been studied, and these units are of great significance due to unhygienic handling. However, there have been some recent studies on the mycotoxins present in food products from Nigeria, especially maize, rice, groundnuts, guinea corn, sorghum, cocoa, and cocoa-based beverages. These study therefore was aimed at studying the food/nutrient values of Abacha in relation to the effects of storage time and fungal biodeterioration of this Abacha samples from different markets and as well studied the aflatoxin contents for better understanding on handling and suspected fungal strains commonly associated with this food product as well as the safety measures to be considered during the storage. It was hypothesized that some fungal are associated with Abacha samples, it is possible that these fungi may or may not be able to produce aflatoxins, this may or not affect the nutrient composition and also, the storage time may have effect on the associated fungal strains.
Materials and Methods
Collection of samples
Abacha samples (100 g each) were purchased from different markets in different location at Aba metropolis, Abia State in Nigeria (Nkwo-ngwa 5.10160N, 7.36816E; Cemetry market 5.09966N, 7.46447E; Aba market 5.09889N, 7.36447E; Ariaria 5.11806N, 7.33250W and New market 5.10003N, 7.37008W) in three replicates per market, which makes sample size of 5 × 2 × 3. The samples were of two types; the freshly prepared samples (1-2 days old) and the stored samples (4 month old). They were separately packaged in sterile polythene bag before taken them to the mycology and pathology laboratory of the department of Botany, University of Ibadan immediately for further experiment.
Characterization of dio-deteriorating fungi
This was done on both collected fresh and stored abacha samples using the method described by Jonathan and Olowalafe [7], fungi that are associated with the food samples were isolated using the medium called Potato Dextrose Agar (PDA). It was prepared according to the manufacturer’s specification, with the addition of 0.5 g streptomycin sulphate (39 g/L) to suppress bacterial growth. The culture medium was then aseptically poured into sterile petri dishes and allowed to solidify. The fungi were isolated from the abacha samples through sterile serial dilution method (10-3 dilution factor). One ml of each of the diluted abacha sample was then aseptically inoculated into the petri dishes containing the PDA. The plates were covered and incubated for 3 to 6 days at 27 ± 2°C. The fungal cultures obtained were subsequently subcultured in order to get pure cultures.
Identification of isolated fungi and percentage incidence
The pure fungal cultures obtained from both fresh and stored samples were identified based on their macroscopic appearance on the culture medium, microscopic morphology and type of asexual spores produced with the aid of photomicrograph as described by Kulwant et al. [8], the microscopic study was done through the use of Olympus BX60 microscope fortified with ultraviolet (UV) light filter band pass opening of above 420 nm wavelength and it is installed with a sigma scan for photographs measurements.
Aflatoxin detection and quantification in ‘Abacha’ samples
The Aflatoxin analysis was carried out on the fresh and dried samples using the method of Thomas et al. [9] and Garrido Frenich et al. [10] using Thin Layer Chromatography (TLC), Aflatoxin B1, B2, G1 and G2 were quantified. 5 grams of dried Abacha were ground respectively into powder, sample digestion was done using saturated sodium chloride and 50 ml hexane and re-reaction cupric carbonate and extract stored in chloroform.
The stored chloroform extract was re-dissolved in 1ml of chloroform and poured into a cuvette of the spectrophotometer and aflatoxin activity was read at 250 nm wavelength. The intensity of the fluorescence produced in spots of the samples was compared with that of the standard aflatoxin spots. The volume of the matching spots of the sample with that of the standard was recorded. The concentration of the aflatoxin in the sample was calculated by the formula
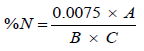 (1)
(1)
Where
S= Volume of each aflatoxin standard, in μl of equivalent intensity to Z μl of sample.
Y= Concentration of each of the aflatoxin standard in μg/ml.
V= Volume of solvent in μl required to dilute final extract.
Z= Volume of sample extract in μl required to give fluorescence intensity comparable to that of S of each aflatoxin standard.
W=Weight of original sample, in gram contained in final sample.
The fresh and stored samples were analysed for their nutrient composition using the method described by the Association of Official Analytical Chemist (A.O.A.C 2008). All the analysis were carried out in three replicates.
The crude protein composition of the sample was determined using the colorimetric technique, Samples were digested using selenium catalyx and concentrated H2S04, minerals were stabilized using potassium tartrate and sodium citrate solutions, Polyvinyl Alchohol and Nesseler Reagent was added to the solution and colour development was read on the spectrophotometer at 460 nm wavelength.
The protein content can be calculated by formula
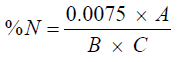 (2)
(2)
Where A=Absorbance from the Spectrophotometer.
B=Weight of the sample.
C=ml of Digest Analysed (i.e. 1 ml).
Percent crude fat was determined through fat free extraction thimble and pug lightly with cotton wool, the soxhlet extraction method. The initial and final weight of the dry soxhlet extracts were compared to quantify % crude fat as follows:
% Fat = W1 – W0 × 100 (3)
Where W1=final weight of flask + fat.
W0=Initial weight of the soxhlet flask.
Percent dry matter and moisture content of the fresh and stored samples were also determined using the formular below,
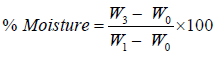 (4)
(4)
Where W0=weight of empty crucible
W1=weight of crucible + sample
W3=weight of crucible + oven dried sample.
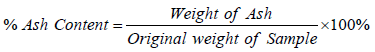 (5)
(5)
Percent crude fibre content of the samples were determined with the use of fibre flask containing 100 ml of 0.255N H2S04, after heating for 1 hr with each sample, 0.313N NaOH was added and heated under reflux for another 1 hour, acetone was then added to dissolve any organic constituent. The extracts were heated and weighed initial and final mass and the percentage fibre was obtained by the formula:
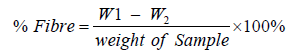 (6)
(6)
While percent carbohydrate content was also measured as;
% Carbohydrate=100 – (% moisture + % crude protein + % crude fat + % ash). (7) [8].
Statistical data analysis
The data generated from these investigations were subjected to analysis of variance (ANOVA) at probability levels of P?0.05 using SPSS version 20. The test of significance was carried out using Duncan?s multiple range test (DMRT).
Results
Occurrence of bio deteriorating fungi in ‘Abacha’ sample from different locations in Aba Nigeria
A total of ten (10) different fungal species were isolated from the stored abacha and they were identified as Aspergillus flavus, A. fumigatus, A. niger, A. ochraceus, A. tamarii, Fusarium sp, Penicillum chrysogenum, Penicillum sp, P. oxalicum, Rhizopus stolonifer as shown in Figure 1. All these fungi are known to cause food spoilage ‘biodeterioration’ however, A. flavus, A. niger, and Rhizopus stolonifer were the most isolated from all the samples across different locations, followed by Fusarium sp. and P. chrysogenum while A. fumigatus, A ochracheus and Trichoderma sp. had the least occurrence (Table 1).
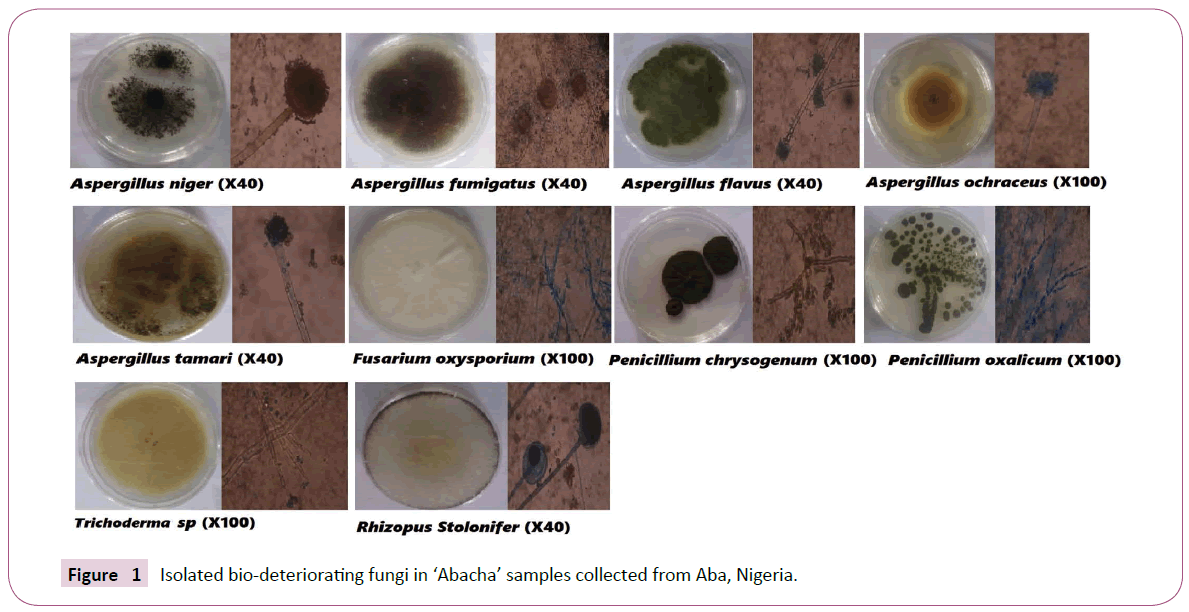
Figure 1: Isolated bio-deteriorating fungi in ‘Abacha’ samples collected from Aba, Nigeria.
| Fungal isolates |
Nkwo-ngwa market |
Cemetry markets |
Ariaria market |
New market |
| Aspergillus flavus |
+ |
+ |
+ |
+ |
| A. fumigatus |
+ |
- |
+ |
- |
| A. niger |
+ |
+ |
+ |
+ |
| A. tamari |
- |
+ |
- |
+ |
| A. ochraceus |
+ |
- |
- |
- |
| Fusarium sp. |
+ |
+ |
+ |
- |
| Penicillium chrysogenum |
+ |
+ |
+ |
+ |
| Penicillium oxalicum |
- |
+ |
+ |
- |
| Trichoderma sp. |
+ |
- |
- |
- |
| Rhizopus stolonifer |
+ |
+ |
+ |
+ |
+ Presence of fungi
-Absence of fungi |
Table 1: Occurrence of fungi in different location.
Furthermore, the fungi incidence in each location differs as the Nkwo-ngwa market had the most fungi incidence followed by Cementary and Ariaria markets while Newmarket had the least (Table 1).
Nutrient compositions of ‘Abacha’
Effect of locations: The nutrient analysis of the samples collected from different location showed variable concentrations as shown in Table 2 below. The crude protein in the sample from Nkwongwa market and Ariaria market are not significantly different (p ≤ 0.05) from one another while there is a significant difference between the percentage crude protein in the samples from Newmarket and cemetery market.
| Source of variation |
% Crude Protein |
% |
% Crude Fiber |
% |
% |
% Carbohydrate |
| Fat |
Ash |
Moisture Content |
| Control |
1.57000c |
0.4550c |
0.5050ba |
0.4000d |
10.6000d |
97.0700a |
| Nkwo ngwa market |
1.6500a |
0.5125a |
0.4950bc |
0.4200b |
11.1650c |
97.1600a |
| Cemetery market |
1.6000b |
0.4900b |
0.4550d |
0.4125cb |
11.1600c |
97.1925a |
| Ariaria market |
1.6450a |
0.4550c |
0.5125a |
0.4300a |
12.0150b |
96.9575a |
| Newmarket |
1.5650c |
0.4375d |
0.4850c |
0.4050cd |
12.1125a |
97.3275a |
Each value is a mean of three replicates. Values in the same column with different letter as superscripts are significantly different by Duncan multiple range test; Confidence interval measured at α0.05
Table 2: Effect of location on the Nutrient compositions of Abacha samples.
The fat content in the samples from the different markets are significantly different (p ≤ 0.05) from each other. The abacha samples from Ariaria markets had the highest crude fibre content while samples from cemetery market had the least. The ash content of the samples from Nkwo ngwa, Cemetery and Newmarkets are significantly different (p ≤ 0.05) from the one from Ariaria market. The highest value for moisture content is found in sample from Newmarket (12.11%) while the lowest value for moisture content is found in the sample from cemetery market (11.16%). There is no significant difference in the value of carbohydrate content across the different markets.
Effect of storage period: The result presented in Table 3 shows the effect of storage period on the nutrient composition of laboratory prepared abacha sample control. The crude protein, fat, crude fibre and ash contents decreased at the fourth month of storage while the moisture content increased at the fourth month. The value of the carbohydrate content increased at the fourth month but it was not significantly different (p ≤ 0.05) from that of the one month old sample.
| Source of Variation |
% Crude Protein |
%
Fat |
% Crude Fiber |
%
Ash |
%
Moisture Content |
% Carbohydrate |
| Control |
1.5700c |
0.4550b |
0.5050a |
0.4000c |
10.6000c |
97.0700a |
| 1 month old |
1.6288a |
0.4863a |
0.4913cb |
0.4250a |
11.0113b |
97.1538a |
| 4 months old |
1.6013b |
0.4613b |
0.4825b |
0.4088b |
12.2150a |
97.1650a |
Each value is a mean of three replicates. Values in the same column with different letter as superscripts are significantly different by Duncan multiple range test; Confidence interval measured at α0.05.
Table 3: Effect of storage period on the Nutrient compositions of Abacha samples.
The mean effect of location, storage period and replicates on the nutrient composition of abacha samples is shown in supplementary Table S1. The results shows that the markets which represent different locations has a high significant effect on all the nutrient compositions except for carbohydrate where it is non-significant. The storage period also has a high significant effect on all the nutrient composition except for crude fiber where it is just significant and carbohydrate where it is non significance while the replicates does not have any significance effect on the all the nutrient compositions.
Aflatoxin analysis of Abacha samples
Effect of locations: The result in Table 4 shows the effects of different location on the aflatoxin concentration of abacha samples. The samples from Cemetery and Nkwo ngwa markets have the highest aflatoxin B1 content while the least is found in the sample from Newmarket. The highest aflatoxin B2 content is found in cemetery market while the least is found in the control sample. Aflatoxin G1 has the highest value in the samples from Ariaria market while aflatoxin G2 has its highest concentration in the samples from cemetery market and Newmarket.
| Source of Variation |
AFB1 (µgkg-1) |
AFB2 (µgkg-1) |
AFG1 (µgkg-1) |
AFG2 (µgkg-1) |
| Control |
0.0025c |
0.0020d |
0.0100c |
0.0100b |
| Nkwo Ngwa market |
0.0048a |
0.0058cb |
0.0150bc |
0.0300a |
| Cemetery market |
0.0048a |
0.0068a |
0.0175ba |
0.0350a |
| Ariaria market |
0.0038b |
0.0060b |
0.0225a |
0.0300a |
| Newmarket |
0.0040ab |
0.0053c |
0.0175ba |
0.0350a |
Each value is a mean of three replicates. Values in the same column with the same letter as superscripts are not significantly different by Duncan multiple range test; Confidence interval measured at α0.05; AFB1: Aflatoxin B1; AFB2: Aflatoxin B2; AFG1: Aflatoxin G1; AFG2: Aflatoxin G2
Table 4: Effect of location on the Aflatoxin concentration of ‘Abacha’ samples.
Effect of storage periods: The effect of storage period on the aflatoxin concentration of abacha samples is shown in Table 5. Aflatoxins B1, B2, G1 and G2 concentration in the 1 month old abacha is significantly different (p ≤ 0.05) from that of the 4 month old abacha samples. The concentration of aflatoxin B1, B2, G1 and G2 increased at the fourth month of storage.
| Source of Variation |
AFB1 (µgkg-1) |
AFB2 (µgkg-1) |
AFG1 (µgkg-1) |
AFG2 (µgkg-1) |
| Control |
0.0025b |
0.0020c |
0.0100b |
0.0100c |
| 1 month old |
0.0030b |
0.0048b |
0.0150b |
0.0263b |
| 4 months old |
0.0056a |
0.0071a |
0.0213a |
0.0388a |
Each value is a mean of three replicates. Values in the same column with different letter as superscripts are significantly different by Duncan multiple range test; Confidence interval measured at α0.05.
Table 5: Effect of storage period on the aflatoxin concentrations in ‘Abacha’ samples.
The results in supplementary Table S2 show the mean effect of location, storage period, and replicates on the aflatoxin concentration in Abacha samples. The markets which represents the locations has a high significant (p<0.05) effect on the aflatoxin B1 and aflatoxin B2 present in the abacha samples while it is nonsignificant for aflatoxin G1 and aflatoxin G2. The storage period has a high significant (p<0.05) effect on the aflatoxin B1, aflatoxin B2 , aflatoxin G1 and aflatoxin G2 content in the samples.
Discussion
This study showed the presence of different fungal species in the stored Abacha samples. The fungi are similar to those reported by Abia et al. [7] and Rubert et al. [8] from shaved cassava, ogbono and tiger-nut respectively. The presence of these fungi species in these food products could be due to open air exposure during sun drying, storage condition or direct contacts in the different markets as reported by Okigbo [7] and Abia et al. [7]. Four fungi genera namely; Aspergillus, Penicillium, Rhizopus, and Fusarium were the most incident fungi with Aspergillus and Penicillum been the most predominant fungi associated with the biodeterioration of abacha samples supporting the findings of Essono et al. [9]. who reported Aspergillus and Pencillium spp. as the common storage fungi. Furthermore, majority of the isolated fungi belong to the genus Aspergillus, thereby supporting the work of Aguoru et al. [10]. That reported Aspergillus species as one of the major contaminants in the environment and produce; therefore, these could be partly responsible for the detected aflatoxins in the abacha samples.
Furthermore, the fungi species isolated from this study increased at the fourth month of storage, and this is in agreement with the work of Fagbohun et al. [11] and Lawal et al. [12] who reported progressive increase in species of fungi in stored sundried plantain chips and coco yam chips respectively. The difference in the occurrence of fungi species in these food products across different locations could as well be as a result of the different method of processing or storage peculiar to each of these locations. The results of the nutrient analysis is similar to the observations of Jonathan et al. [13] and Ogunsina et al. [14] when they worked on stored gbodo and ogbono respectively. Abacha samples were significantly different (p<0.05) in their nutrient composition.
The studied samples showed high in moisture (10.6% to 12.21%) and carbohydrate (96.95% to 97.95%) contents but lower protein (1.57% to 1.63%), fat (0.44% to 0.49%), fibre (0.48% t0 0.51), and ash (0.40% to 0.43%) contents but there which decreased with storage time probably due to microbial growth (including the fungi)in the abacha coupled with microbial degradation [15-21]. Interestingly, an increase in the moisture content of the samples was observed as the storage period increased; this may be due to the moisture absorbed from the atmosphere during storage. The quantity of crude protein, fat and crude fibre in these samples decreased with an increase in the storage period, similar observation have been earlier reported by kolapo and sanni [22] and kolapo et al. [23] for soya-bean, daddawa and some selected oil seeds cakes respectively.
Varying levels of aflatoxin concentrations (AFB1, AFB2, AFG1 and AFG2) were detected in the studied food samples that varied according to location and storage period; the aflatoxins were probably produced by the aflatoxigenic fungi species observed. The increasing concentration of the aflatoxins in the stored samples with the storage period corroborated the observations made by Kaaya and Eboku [24], Adebayo-tayo et al. [25] and Saleemullah et al. [26] for cassava chips, ogbono and cereals/ nuts respectively. This study together with those of Rahimi et al. [27-32], Jonathan et al. [33] and Etebu [34-37] affirm that storage method and time are of immense importance in the control of food intoxication.t.
Conclusion
The public health effect of aflatoxin concentration of ‘Abacha’ or possible incidence or prevalence of any ‘mycotic’ fungal infection(s) on the host community was not studied in this report, it is however observed from this study that the aflatoxin concentrations in the studied food product were within the permissive level of 20 μg/mg for adults as stipulated in most countries. However, increase in the storage period led to an increase in fungi load and aflatoxin concentration, thus resulting to a drop in the nutritional compositions of the studied food products, further storage may increase the concentration above the permissive/tolerance level. Furthermore, the different locations from which the food products were collected showed a significant effect on the fungi load and aflatoxin concentration which might be as a result of different use of storage methods, it is therefore necessary to prevent fungal growth in stored food in other to prevent aflatoxin contamination and this could be achieved through the application of strict hygienic measures during processing and storage periods.
Ethical Issues
This study original, not previously published and not under consideration for publication elsewhere. All the author participated in the study to a significant extent. The work does not involve the use of human or animal participants.
Patient Anonymity and Informed Consent
This study does not involve the use of human participant.
Conflicts of Interest
The authors declare no conflict of interest on this research paper.
20623
References
- Abia WA, Warth B, Sulyok M, Krska, R, Tchana AN (2013) Biomonitoring of mycotoxin exposure in Cameroon using a urinary multi-biomarker approach. Food and Chemical Toxicology 62: 927-934.
- Aguoru CU, Okoli BE, Olasan JO (2014) Phytogeography of the genus Sesamum L. (Pedaliaceae) in Nigeria, West Tropical Africa Scient J Crop Sci 3: 115-122.
- Association of Analytical Chemistry (AOAC) (2008) Official Methods of Analysis, Association of Official Analytical Chemist, Arlington, Va, USA 20: 108.
- Bankole SA, Ogunsanwo BM, Eseigbe DA (2005) Aflatoxins in Nigerian dry-roasted groundnuts, Food Chemistry 89: 503-506.
- Beltrin E, Ibanez M, Sancho J, Cortes M, Yusa V (2001) UHPLC-MS/MS highly sensitive determination of aflatoxins, the aflatoxin metabolite M1 and ochratoxin A in baby food and milk. Food Chemistry 126: 737-744.
- Bendech A, Chauliac M, Rerolle GP, Kante N, Malvy DJ (2000) Les Enjeux du consommateur en milieu urbain a Bamako. Journal de Sante Publique 12: 45-63.
- Ekwu FC, Ngoddy PO, Anekwe CO (2009) ‘Abacha’ making qualities of selected cassava cultvars. Nigerian Food Journal 22: 49-55.
- Ekwu FC, Uvere PO, Chinwero OM (2012) Quality of ‘Abacha’ from Fresh Cassava Roots and Dried Chips. Nigerian food journals 30: 124-130.
- Ekwu FC, Ngoddy PO, Uvere PO (2013) Effect of processing on the quality of flour, abacha slices and its flour derived from cassava (Manihot esculenta Crantz) TMS 97/4779. African Journal of food science 8: 476-483.
- Essono G, Ayodele M, Akoa A, Foko J, Filtenborg O (2008) Aflatoxin-producing Aspergillus spp. and aflatoxin levels in stored cassava chips as affected by processing practices. Food Control 20: 648-665.
- Etebu E, Bawo D (2011) Fungal quality and phytochemicals of Irvingia gabonensis (Aubry-Lecomte ex O’Rorke) kernels sold in Yenagoa metropolis of Bayelsa State, Nigeria. J Biol Agric Healthcare 2: 41-50.
- Fagbohun ED, Abegunde OK, David OM (2010) Nutritional and mycoflora changes during storage of plantain chips and the health implications. Journal of Agriculture Biotechnology and Sustainable Development 2: 61-65.
- Fandohan P, Zoumenou, D, Hounhouigan DJ, Marasas, WF, Wingfield, MJ, et al. (2005) Fate of aflatoxins and fuminosins during the processing of into food products in Benin. Int J Food Microbiol 98: 249-259.
- Garrido Frenich A, Martínez Vidal JL, Romero-González R, Aguilera-Luiz MM (2009) Simple and high-throughput method for the multimycotoxin analysis in cereals and related foods by ultra-high performance liquid chromatography/tandem mass spectrometry. Food Chemistry 117: 705-712.
- Jonathan SG, Olowolafe TB (2001) Studies on nutrient contents and microorganisms associated with dodo Ikire a plantain snack from Western Nigeria. NISEB J 1: 27-30.
- Jonathan SG, Adeniyi MA, Asemoloye MD (2015) Fungal biodeterioration, aflatoxin contamination and nutrient value of ‘aadun’. Researcher 7: 26-32.
- Jonathan SG, Ajayi L, Omitade Y (2011) Nutritional compositions, fungi and aflatoxins detection in stored ‘gbodo’ fermented (Dioscorea rotundata) and ‘elubo ogede’ fermented (Musa paradisiaca) from South Western Nigeria. African Journal of Food Science 5: 105-110.
- Jonathan SG, Asemoloye MD, Abe A, Olawuyi OJ, Aina D (2016) Food value, fungi and aflatoxin detection in stored ‘orunla’ Abelmoschus esculentus L. (Moench) from Ibadan, Nigeria. Researcher 8: 7-18.
- Jonathan SG, Adeniyi MA, Asemoloye MD (2016) Nutrient value, fungal biodeterioration, and aflatoxin contamination of suya spices a Novel Nigerian Indigenous Snacks. Hindawi – Scientifica 46: 202-236.
- Kaaya AN, Eboku D (2010) Mould and Aflatoxin Contamination of Dried Cassava Chips in Eastern Uganda: Association with Traditional Processing and Storage Practices. Journal of Biological Sciences 10: 718-729.
- Kaaya AN, Kyamuhangire W, Kyamanywa S (2006) Factors affecting aflatoxin contamination of harvested maize in the three agroecological zones of Uganda. J Appl Sci 6: 2401-2407.
- Kolapo AL, Oladimeji GR, Ifejika AI, Osakwe OE, Eyitayo IR (2012) Aflatoxin, Nutritive Values and Microbiological Status of Stored Cakes of Some Selected Nigerian Oil Seeds. Global Journal of Science Frontier Research Agriculture & Biology 12: 1.
- Kolapo AL, Sanni MO (2007) Biochemical changes of Soyiru (fermented soybean) with Storage. Agricultural and Food Science Journal of Ghana 6: 471-483.
- Kulwant S, Jens C, Frisvad Thrane UF, Malhur SB (1991) An illustrated manual on identification of some seedborne Aspergilli, Fusaria, Penicillia and their mycotoxins. 1 Edition, Danish Governorate, Institute of Seed Pathology for Developing Countries Ryvangs Alle 78.
- Lawal OU, Fagbohun ED, Olajide HA (2012) Nutritive value and mycoflora of sun dried cocoyam chips during storage. International Journal of Agronomy and Agricultural Research 2: 1-7.
- Lopez-Garcia R, Park DL (1998) Effectiveness of post-harvest procedures in management of mycotoxin hazards. In: Mycotoxins in agriculture and food safety. New York, Marcel Dekker 22: 407-433.
- Marin S, Ramos AJ, Cano-Sancho G, Sanchis V (2013) Mycotoxins: Occurrence, toxicology, and exposure assessment. Food and Chemical Toxicology 60: 218-237.
- Mutegi CK, Ngugi HK, Hendriks SL, Jones RB (2009) Prevalence and factors associated with aflatoxin contamination of peanuts from Western Kenya. International Journal of Food Microbiology 130: 27-34.
- Njoku, BA, Banijo E (2006) Physico-chemical properties of pre-cooked cassava (Manihot esculenta Cranz) flour prepared through adaptation of traditional process. Nigerian Food Journal 24: 98-106.
- Okigbo RN (2003) Fungi Associated with Peels of Post-Harvest Yams in Storage. Global J Pure App Sci. 9: 19-23.
- Park DL (2002) Effect of processing on aflatoxin. Adv Exp Med Biol 504: 173-179.
- Rachaputi NR, Wright GC, Kroschi S (2002) Management practices to minimize pre-harvest aflatoxin contamination in Australian groundnuts. Australian J Exp Agric 42: 595-605.
- Rahimi E, Bonyadian M, Rafei M, Aazemeini HR (2010) Occurrence of aflatoxin M1 in raw milk of five dairy species in Ahvaz, Iran. Food and Chemical Toxicology 48: 129-131.
- Rubert J, Sebastià N, Soriano JM, Soler C, Mañes J (2011) One-year monitoring of aflatoxins and ochratoxin A in tiger-nuts and their beverages. Food Chemistry 127: 822-826.
- Saleemullah AI, Khalil IA, Shah H (2006) Aflatoxin contents of stored and artificially inoculated cereals and nuts. Food Chemistry 98: 699-703.
- Thomas FR, Eppley M, Trucksess MW (1975) Rapid screening method for aflatoxins and zearalenone in corn. J Assoc off Anal Chern 58: 114-116.
- Yu J, Ahmedna M, Goktepe I (2004) Effects of processing methods and extraction solvents on concentration and antioxidant activity of peanut skin phenolics. Food Chemistry 90: 199- 206.






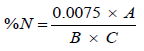 (1)
(1)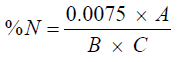 (2)
(2)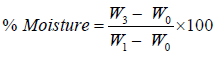 (4)
(4)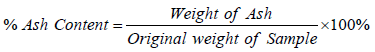 (5)
(5)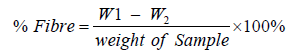 (6)
(6)