Keywords
Osteoarthritis, Diacerein, Diclofenac, Visual analog scale, Western On- tario and McMaster Universities Osteoarthritis Index, Lequesne impairment index
Introduction
Osteoarthritis (OA) is the most common joint disease of humans both in the western world as well as in India. It affects synovial joints and is characterized by progressive loss of articular cartilage with subchondral bone remodeling, inflammation of synovial membrane, subchondral bone scleroris and osteophyte formation [1,2]. OA present clinically with fluctuating joint pain, swelling, stiffness, and loss of mobility, which increase in severity with disease progression. Non-pharmacological treatments of OA include measures to reduce joint load, regular aerobic, muscle strengthening and range of motion exercises.0, maintaining weight at lower levels, knee brace, medial taping of patella, wedged soles, thermal modalities and patient education etc. Pharmacological therapy includes topical and systemic use of nonsteroidal anti-inflammatory drugs (NSAIDs), including selective cyclooxygenase 2 (COX-2) inhibitors, opioids and intra-articular steroids. Since most of these are meant only for symptomatic improvement, there is lot of research going on in the field of disease modifying agents that can aid cartilage repair. Diacerein, glucosamine, bisphosphonates and cytokine inhibitors are some of these drugs. It is now widely accepted that interleukin-1β (IL-1β) plays a key role in inflammation, cartilage breakdown, chondrocyte apoptosis and bone remodeling in OA [3-8]. Diacerein, an anthraquinone derivative, is a slow acting drug in OA [9]. In vitro studies have shown that apart from inhibiting IL-1β, diacerein also stimulates the production of cartilage growth factors such as transforming growth factor β [10]. In animal models of OA, diacerein has been shown to significantly reduce cartilage degradation as compared to untreated animals [11-13]. Since it does not inhibit prostaglandins [14], diacerein does not have a deleterious effect on the upper gastrointestinal mucosa [15]. In clinical trials, it has been shown to significantly decrease OA symptoms [16-23], and a 3-year study showed that it has structure-modifying effects [24]. Diacerein was launched in Indian market in 2006 and aggressively marketed as the new wonder cure for OA. The data available on Indian population at the time of drug launch was inadequate. Moreover, there is no data available on comparative change in quality of life among diacerein users. Therefore, this study was planned to investigate the effect on quality of life, efficacy and adverse effect profile and carry-over effects of diacerein and diclofenac given alone or in combination in Indian osteroarthritic population.
Methods
Study Design
A single-blind, randomized, parallel, comparative model was designed for the study. The study was conducted in accordance with the Helsinki declaration and subsequent revisions and with good clinical practice. The study was done during the period from Dec 2008 to Feb 2010. Patients attending the out patient department (OPD) of Orthopedics, Lok Nayak Jai Prakash (LNJP) hospital were enrolled during the period from December, 2008 to September, 2009. The study was approved by the departmental scientific review board and the ethics committee of Maulana Azad Medical College. A written informed consent was taken from the patients inducted into the trial. In order to facilitate follow-up, patients living within 15 kilometers of the hospital premises were recruited. Female pre-menopausal patients were screened for pregnancy by urine pregnancy kit and were cautioned against getting pregnant during the study period. They were asked to take adequate precautions against pregnancy during this period. Oral contraceptive pills were not allowed during the study period and female subjects were encouraged to use barrier methods of contraception or intra-uterine devices. Patients were asked if they can come to the OPD every 15 days for the next 16 weeks. In case of their inability, they were not included in the study but were given treatment as per the standard guidelines. After meeting the inclusion and exclusion criteria and signing the informed consent form, patients were randomized into three groups who received diacerein (50mg twice daily) or diclofenac (75mg twice daily) or both diacerein (50mg once daily) and diclofenac (75mg once daily) respectively (Fig.1). Randomization was done electronically using the website www.randomizer.org. Block randomization was done with 3 patients in each block such that there is equal number of patients in all the groups throughout the study. Each allocation was concealed and kept in separate opaque envelope to be opened one by one as subjects got randomized and enrolled. All the groups also received omeprazole 20mg in addition. Following 12 weeks of therapy, patients were followed up for another 4 weeks. Each patient was followed up for 17 weeks from randomization till the end of the follow up period (1 week washout period, 12 weeks of treatment and 4 weeks of follow up). Detailed physical examination including knee society score (KSS),[27] subjective assessment using visual analog scale (VAS) score after 20m walk, Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) [28] and Lequesne Impairment Index (LII) [29] and quality of life assessment using SF-36 form [30] was done following randomization. The use of NSAIDs, if any, was stopped one week prior to the start of treatment. Patients were allowed the use of acetaminophen (1000mg) as a rescue medication in case of intolerable pain. The patients were given a calendar where they were asked to circle the dates when they had to use the rescue medication. They were asked to bring the calendar at each visit to calculate pain free days per month. After every 4 weeks, they underwent evaluation of symptomatology and physical examination. Patients were asked to fill up WOMAC score, Lequesne index and KSS. Liver function test, renal function test and routine hemogram were also performed every 4 weeks. At the end of 12 weeks of treatment, patients are asked to fill up SF-36 form. After 12 weeks, the treatment was stopped and patients observed for 4 weeks. Patients were also evaluated at each month with VAS score after 20m walk. During the study, patients were not allowed to undergo physical therapy, ultrasonic therapy or to apply any topical gels over the study knee joint. However once the medication period was over, patients were encouraged to start these therapies during their drug free period and beyond.
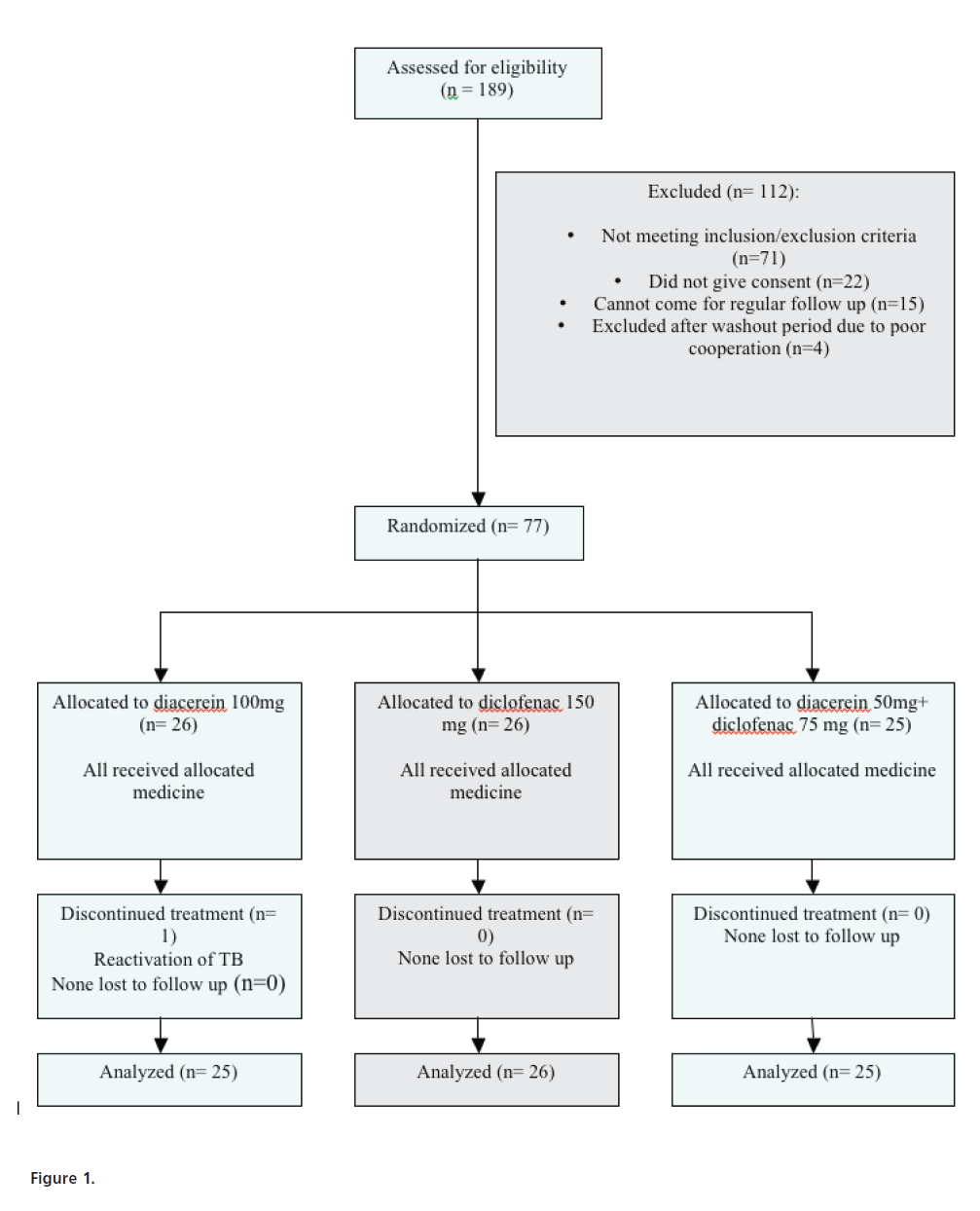
Figure 1:
Subjects
Patients were eligible for the study if they were between 35 and 75 years of age and had tibiofemoral OA according to the modified American College of Rheumatology criteria [25] with a radiologic score of II, III or IV on the Kellgren/Lawrence scale [26]. In cases of bilateral OA, only the more painful knee was assessed.
Exclusion criteria were secondary knee OA, accompanying hip OA, intra-articular or systemic corticosteroid treatment or arthroscopic procedures within 6 months prior to study start, diacerein treatment within 6 months prior to study start, current treatment with antidepressants or tranquilizers, primary painful inflammatory conditions of the knee (e.g., rheumatoid arthritis, gout etc.), severe heart, hepatic (transaminase levels ≥2.5 times the upper limit of normal) and/or renal (serum creatinine ≥1.8 mg/dl or proteinuria of 2+ on >1 test) disease, severe gastrointestinal disorders (ulcer, hemorrhage etc.), pregnancy, lactation and severe articular inflammation as confirmed by physical examination (e.g. finding severe joint effusion).
Efficacy evaluation
The primary and secondary efficacy parameters were assessed at baseline and after every 4 weeks for 16 weeks. The primary efficacy end point was the change from baseline in 100mm VAS score after 20 meters walk (Table 1). The secondary efficacy variables were WOMAC total score, pain sub-score, joint stiffness sub-score, physical function sub-score, LII, KSS, acetaminophen intake (number of tablets taken per day), presence of swelling of soft tissue, tenderness and crepitations of the target knee joint assessed by palpation along the joint line, knee circumference and the quality of life.
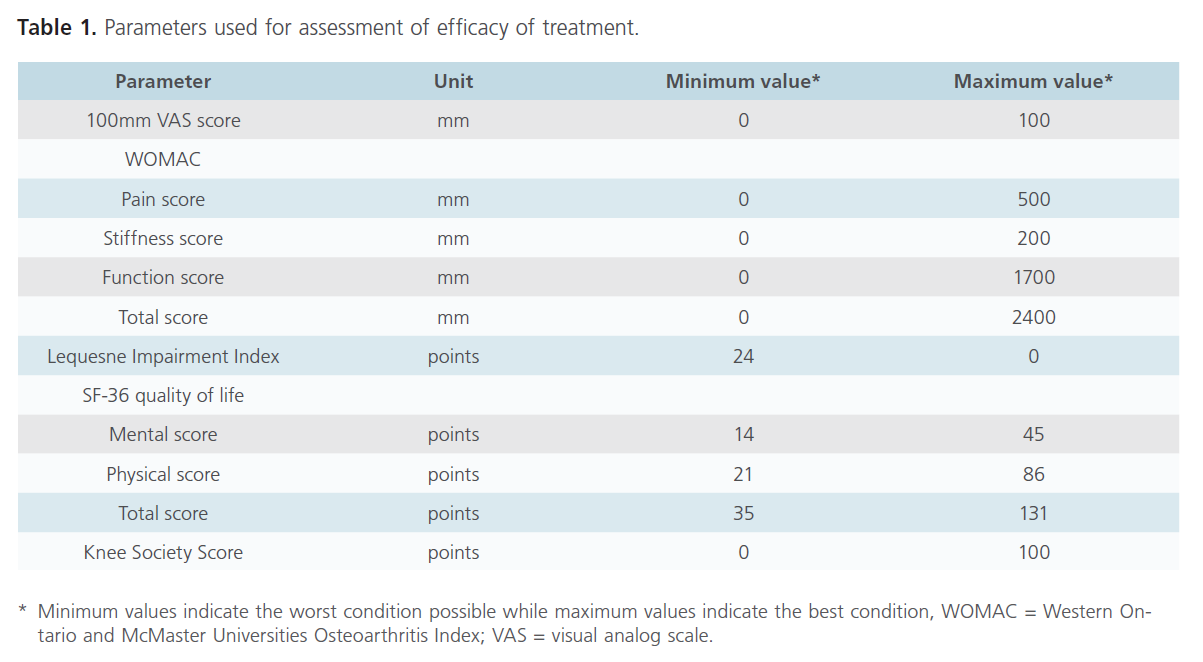
Table 1: Parameters used for assessment of efficacy of treatment.
Safety evaluation
Comprehensive hematological measurements and clinical chemistry studies (including measurements of serum bilirubin, serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, triglycerides, urea and creatinine) were carried out on blood samples obtained at baseline and at the end of the treatment. All adverse events reported by the patients at study visits were recorded. A standardized question (‘have you experienced any discomfort with this medication?’) was asked to each patient regarding adverse effects experienced.
Compliance
To ensure compliance, patients were asked to bring blister packs of the drugs on each on-treatment visit and the number of unused drugs was counted. Patients were motivated by the prescribing doctor on each visit. Follow up visits were made convenient by highlighting their OPD sheets for quick recognition and bypassing the queue. Subjects who missed follow up visits were reminded on telephone. Acetaminophen intake was checked by counting the tablets returned at each visit and reviewing intake information recorded in the calendar provided to the subjects.
Statistical Methods
The three groups were compared using analysis of variance whereas intra-group comparison was done using paired T-test. The categorical values were compared using chi-square test. A p value of less than 0.05 was taken as significant (95% Confidence Interval-CI). The software used for all statistical analysis was SPSS ver.10.0 and was done using the services of a trained statistician.
Results
A total of 189 subjects were screened from December 2008 to September 2009. Seventy one subjects were excluded as they did not meet inclusion criteria or fell in the exclusion criteria. Twenty-two subjects did not give consent for enrollment in the study and 15 withdrew as they expressed their inability to come for regular follow up every 2 weeks.
Of the 81 subjects who entered the study, 4 subjects were excluded from the study as they were assessed to be uncooperative, non-motivated and/or negligent and were prone to discontinue from the study midway. The remaining 77 subjects completed the study with 26 subjects in diacerein group, 26 patients in diclofenac group and 25 patients in combination (diacerein + diclofenac) group. One subject in diacerein group suffered a serious adverse event and discontinued from the study after 8 weeks of treatment. He was followed up as per protocol and was not included in the efficacy analysis. Thus, 76 subjects were finally included in the efficacy analysis (Fig.1).
Demographic and baseline clinical characteristics of patients are shown in Table 2. The 3 treatment groups were similar with regard to demographic data and baseline clinical characteristics.
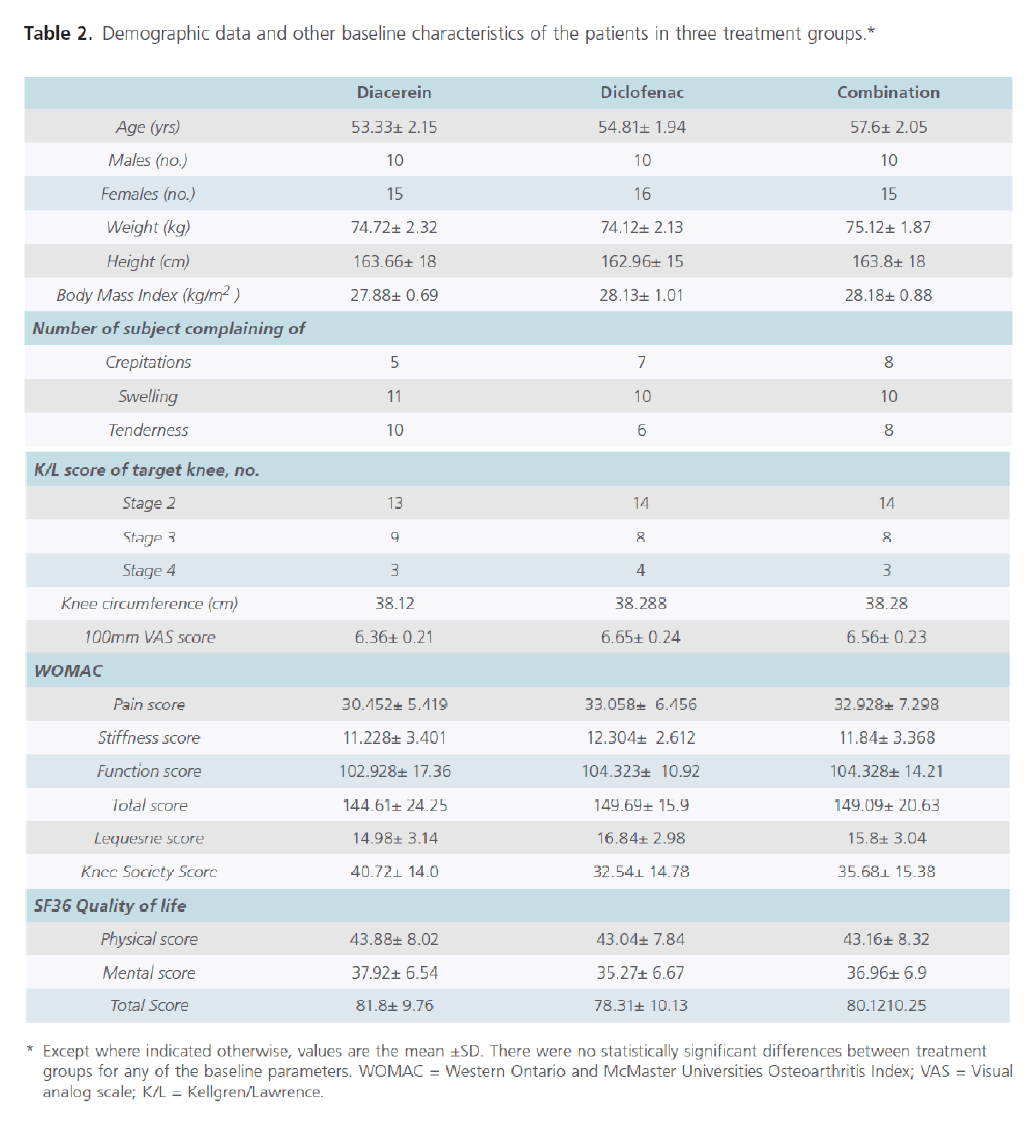
Table 2: Parameters used for assessment of efficacy of treatment.
Safety
Several minor adverse events were reported by the subjects during the study period. The incidence of diarrhea and urine discoloration was significantly higher in diacerein treated group (60% and 40% respectively) as compared to diclofenac group (7.7% and 3.8% respectively). The incidence of epigastric pain and rash in extremities was significantly more common in diclofenac treated group (57.7% and 42.7% respectively) than diacerein group (8% each).
Laboratory investigations revealed that serum cholesterol level was significantly raised in diacerein treated group as compared to diclofenac group in week 4, 8 and 12. Similarly alanine transaminase (ALT) activity was significantly raised in diacerein treated group in week 4 when compared to diclofenac treated group. However, at week 8, the ALT levels came down to normal value.
One patient randomized to diacerein arm of the study developed reactivation of tuberculosis. Past history revealed that he had been treated for pulmonary tuberculosis (TB) for 6 months and declared as cured. Sputum smear examination and BACTEC-460 was done to confirm it before enrollment in the trial. Patient started complaining of several constitutional symptoms suggestive of TB after 2 months of therapy with diacerein. Sputum smear examination revealed acid fast bacilli (AFB). Patient was taken off the diacerein and anti-TB drugs re-instated. After 6 months of therapy patient became sputum-smear negative for AFB. The case was reported to Central Drugs Standard Control Organization (CDSCO) via national pharmacovigilance program because the investigators believed that this re-activation of TB might be related to diacerein.
Efficacy
The primary efficacy parameter, VAS score decreased significantly in all the groups when compared to the baseline values of each group. The difference between diacerein and diclofenac treatment was found to be statistically significant (p<0.05) only at week 16. Similarly, the difference in scores between combination treated subjects and diclofenac treated subjects was statistically significant (p<0.05) at week 16. (Fig.2) Intra-group comparison revealed that WOMAC pain, stiffness and function sub-score as well as total WOMAC scores were significantly different from baseline score in each group. The scores progressively decreased in all the groups till week 12. In diacerein and combination group, reduction in WOMAC pain, function and total score was maintained at follow up period (week 16) whereas WOMAC stiffness score increased at week 16. In subjects treated with only diclofenac, all WOMAC scores tend to increase at week 16 (Table 3). Inter group comparison suggested that diacerein was more effective than diclofenac at week 12 (p<0.001, 0.05 and 0.01 for WOMAC pain, function and total score respectively). This beneficial effect of diacerein was maintained at follow up period i.e. at week 16 (p<0.001). There was no difference in WOMAC stiffness score between diacerein and diclofenac groups at any point of time. Patients treated with combination of diclofenac and diacerein group did not show statistically significant difference in any WOMAC score as compared with diclofenac or diacerein group at the end of the treatment period (week 12). After 4 weeks of follow up, combination treatment group had significantly reduced WOMAC pain, function and total scores as compared to diclofenac treated patients (p<0.05), however WOMAC pain and total scores were much higher than diacerein group at this time (p<0.05). At week 16, there is no statistically significant difference in WOMAC stiffness score of any treatment group (Table 3).
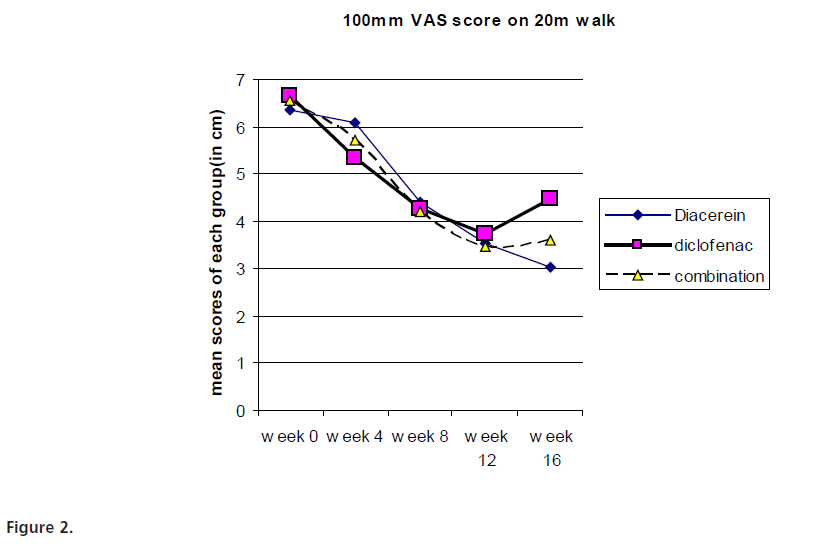
Figure 2:
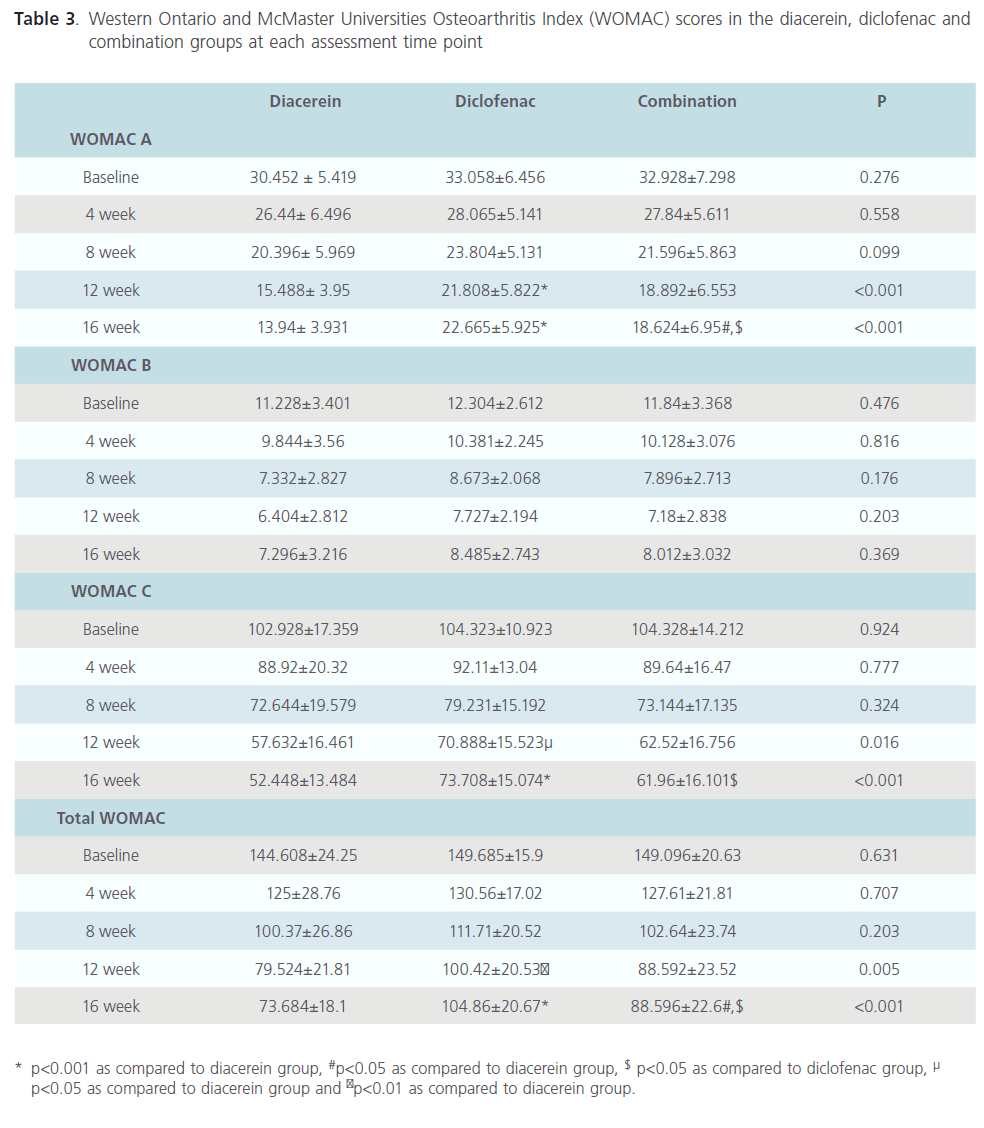
Table 3: Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores in the diacerein, diclofenac and combination groups at each assessment time point
Intra-group analysis of each group revealed that the change in LII, KSS and acetaminophen consumption were significantly different when compared to corresponding baseline scores (Table 4). At the end of the treatment period i.e. 12 weeks, no significant difference was observed in the LII and KSS among different treatment groups, however acetaminophen consumption was significantly reduced in diacerein group as compared to diclofenac group (p<0.05). At the end of the follow up period, diacerein was found to be significantly better in terms of LII, KSS and acetaminophen consumption as compared to diclofenac (p<0.001). Patients treated with combination of diacerein and diclofenac had significantly better KSS than diclofenac treated patients (p<0.05) at the end of follow up period (Table 4). Statistically significant difference was not found for knee circumference at any point of time in different treatment groups (Table 4).
SF-36 quality of life questionnaire was administered to the subjects once at the beginning (week 0) and again at the end of the study (week 16). It comprised of SF-36 physical and mental health. Intra-group analysis of each group revealed that the change in each score (physical health, mental health and total score), when compared to baseline score was significantly different (p<0.0001). However, the scores were not statistically significant when inter-group comparisons were made (Table 4).
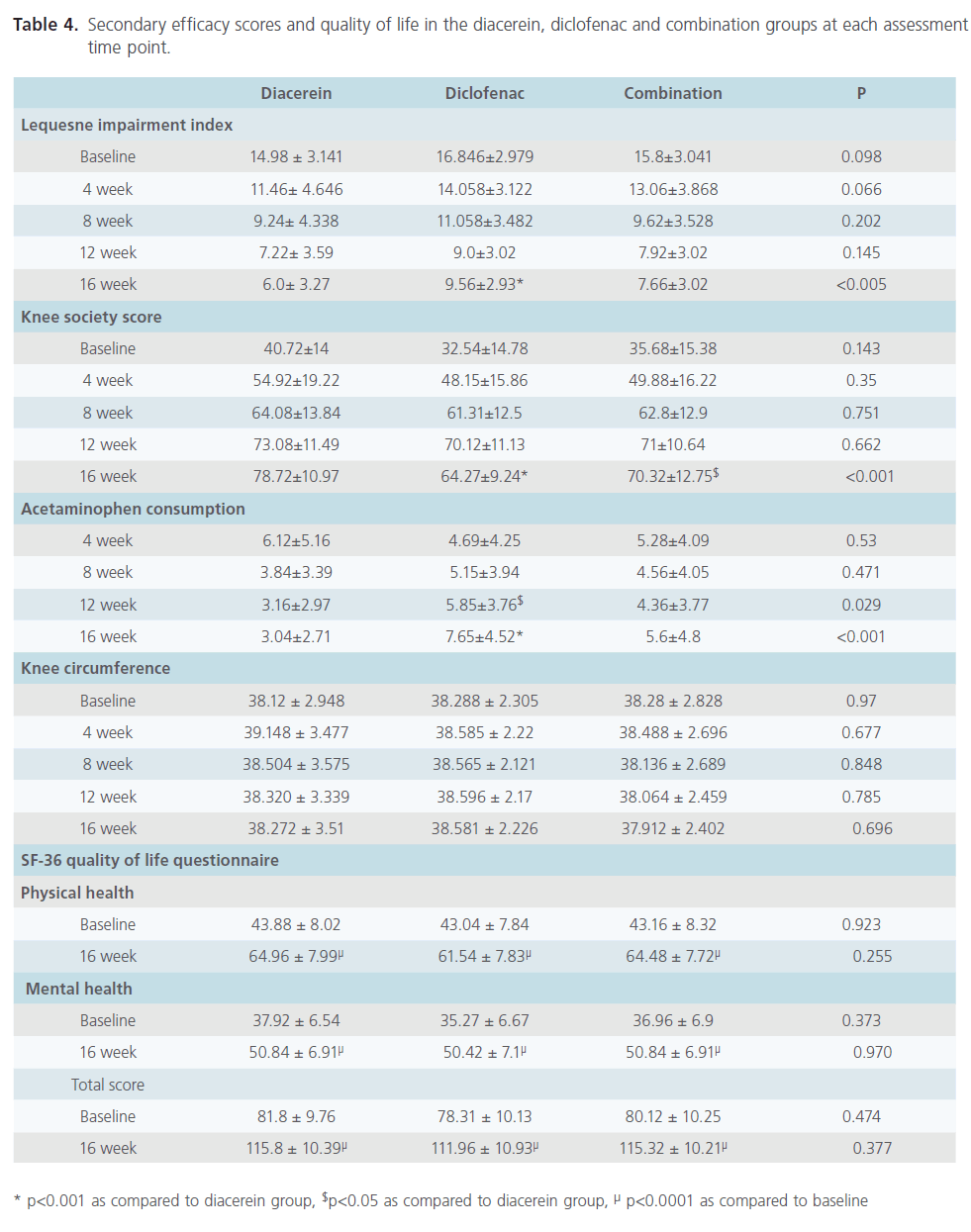
Table 4: Secondary efficacy scores and quality of life in the diacerein, diclofenac and combination groups at each assessment time point.
There was no significant difference in the number of subjects complaining of knee swelling in different treatment groups at baseline but significantly higher number of patients complained of knee swelling in the diclofenac group at week 16 as compared to diacerein treated group (p=0.011). Significantly higher number of patients complained of tenderness in knee joint in diacerein group as compared to diclofenac group at week 4 (p=0.045) but this difference was abolished at follow up period i.e. at week 16. There was no difference among patients complaining of crepitations of movement in different groups at various time intervals(Table 5).
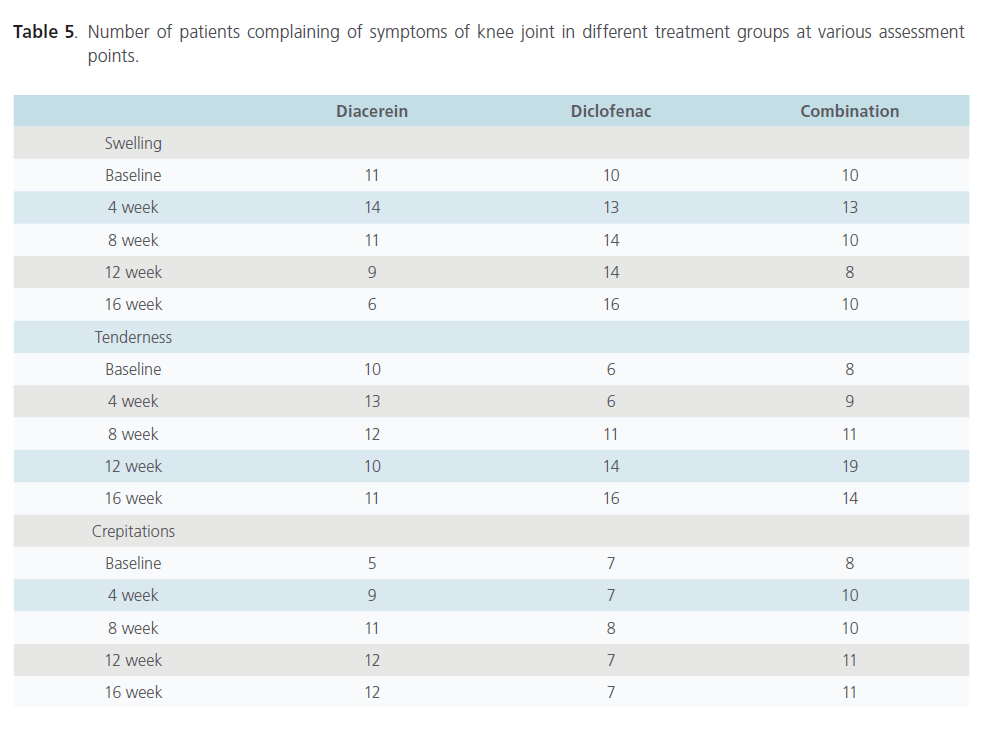
Table 5: Number of patients complaining of symptoms of knee joint in different treatment groups at various assessment points.
Discussion
OA is the most common type of arthritis [31]. Its high prevalence, especially in the elderly, and the high rate of disability make it a leading cause of disability in the elderly. Aging of western populations and the high incidence of obesity are the two major risk factors increasing the prevalence of OA.
Though the Indian population is relatively young but according to sheer numbers, the aged population in India is much bigger than that of western counterparts [32].
While most western literature state that the radiographic evidence of OA is rare in individuals under 40 years of age [31, 33], the present study found that there are several patients coming with symptoms and radiographic evidence at the age of 35 to 38 years.
Diacerein has been studied in western population, its efficacy and safety compared to standard treatment regimens. It has been shown to be as efficacious as standard NSAIDs like diclofenac and in addition, has been shown to possess carry-over effect. This leads to less analgesic consumption after stopping diacerein [18, 20, 24]. Meta-analysis and systematic review of the studies done reveal that there is a small but significant benefit of diacerein over NSAIDs because of its analgesic action and carry-over effect [33-34]. The present randomized open label study was designed to confirm these findings in Indian population.
The primary efficacy criterion was the change in 100mm VAS scale after the patient was asked to take a 20m walk. Zheng et al (2006) compared diacerein (100mg/day) with diclofenac (75mg/day) for OA of knee for a period of 12 weeks [30] and observed that the pain score, using the VAS scale, decreased with both diacerein and diclofenac at the end of 12 week period. However the carry over effect observed with diacerein was not seen in the diclofenac treated group [30]. These findings are similar to that seen in our study where in the VAS score was not significantly different between the 3 treatment groups at 12 weeks. However, the same was significantly different in patients treated with diacerein alone or in combination with diclofenac, although the dose in the combination group was half of what was used by Zheng et al suggesting a significant additive effect of the two drugs. However, the carry over effect at 16 weeks was greater when diacerein was used alone. These findings are similar to another study conducted by Tang et al (2004) [35].
Among the secondary end-points assessed were WOMAC score, LII, KSS and acetaminophen consumption as rescue medication.
The WOMAC score consists of three sub-scores namely pain, stiffness and function sub-scores. In their study, Pelletier et al (2000) [20] compared three doses of diacerein (50, 100 and 150 mg per day) with placebo and observed significant improvement in the WOMAC score with 50 mg and 100 mg per day doses when compared to placebo treatment. However, they were unable to show any significant improvement in the VAS score in these patients. In the present study a statistically significant difference in the WOMAC score was observed at week 16. The order of improvement in the WOMAC score was diacerein having greater score than the group given both diacerein and diclofenac albeit in 50% dose which in turn was better than diclofenac suggesting once again the carry over effect of diacerein. The improvement was significant in the pain and function sub-score as well as the total WOMAC score. In the study by Pelletier et al (2000), diacerein scored significantly better in stiffness and function sub-score as well as total score [20].
Yet another tool for assessment of joint functioning in patients of OA since 1994 [16] is the LII. Several studies have used this index [16,21,24,36,37]. Most of these studies have observed an improvement in the score when the treatment group is compared to the placebo treated group. In one of the initial studies [16] when tenoxicam was compared to diacerein, the former improved the Lequesne score significantly. In the present study, it was observed that there was a significant improvement in the score at week 4 in the patients treated with diacerein alone. The score improved at week 16 in the groups treated with diacerein alone and when given in combination to diclofenac. This once again demonstrates the carry over effects in the improvement of diacerein treated subjects.
The third assessment was made using the KSS. This makes an objective assessment of the affected joint. As in the previous assessment tools, the groups treated with diacerein alone or in combination with diclofenac did show statistically better score, than in the group treated with diclofenac alone.
Another method of assessment of efficacy used in several trials is the compilation of the extent in the use of rescue medication. Acetaminophen has been used in several studies [16,35] as a rescue medication. Nyugen et al (1994) in their study with diacerein observed that in the patients administered diacerein along with tenoxicam, the requirement of acetaminophen as a rescue medication was significantly less than when either of the drug was used alone [16]. In the present study, it was observed that at week 12, patients receiving diacerein required significantly fewer acetaminophen tablets as rescue medication compared to patients receiving diclofenac. This difference was extended at week 16 when the group receiving diacerein and diclofenac combination consumed lesser rescue medication than the group receiving diclofenac alone; yet once again indicating a carry over effect of this drug. Similar observation was made by WJ Zheng et al (2006) [38].
SF-36 quality of life questionnaire is a general form to measure the quality of life. None of the previous studies had done any quality of life assessment of OA patients on diacerein therapy. Even though there was significant change in the quality of life when intra-group comparison was made, present study found that there was no significant difference among the three groups with regard to quality of life. Since, there has not been a single study comparing the change in quality of life among patients taking different drugs for OA, we can only speculate on the reasons for such results. We propose that since all the patients received equally good care and attention from the physicians, the overall change in quality of life was similar in all the groups regardless of the group in which they were allocated. We would like to compare the scores with those of patients who were not enrolled in the study and see if our hypothesis holds true or not [39].
Regarding side effect profile, other studies demonstrate that diarrhea was the most frequent cause of drop outs among all comparison groups. The severity of diarrhea was mild-to-moderate and occurred within the first two weeks of the treatment. In present study the incidence of diarrhea was 13/25 in diacerein group which matches roughly with the 42% rate seen in other studies. Rhein, the chief metabolite of diacerein, has been found to have laxative properties.
A hypothetical explanation is that since diacerein has been shown to be capable of inducing prostaglandin synthesis, it may be that a local increase in prostaglandins can lead to an increase in gut motility and thus to diarrhea [40]. The second most prevalent adverse effect without clinical relevance was discoloration of urine (25% in the diacerein group versus 1.7% in the placebo group in pooled analysis of 6 studies) [34]. However, as these studies reveal, there is no alteration in renal function in present study too. Epigastric pain and rashes were more common among diclofenac only users while diarrhea and discoloration of urine was found more commonly among diacerein only users.
The adverse events associated with diclofenac are well known. Both diacerein and diclofenac are associated with increase in ALT levels. The raised levels came down within 8 weeks of use and were within normal limits by the time of withdrawal of the drugs. Serum cholesterol was found to be raised in the diacerein group after 4 weeks of use. It remained at raised levels till week 16. Diacerein has never been related to increase in serum cholesterol level in previous studies. However, it has been found, in in vitro studies that deficiency of interleukin-1 receptor antagonist (IL-1Ra) deteriorates cholesterol metabolism upon consumption of an atherogenic diet in animal studies. The levels of total cholesterol in IL-1Ra deficient mice were significantly increased, and the start of lipid accumulation in liver was observed earlier when compared with wild type mice [41].
The only major adverse event in the entire study was the re-activation of tuberculosis (TB) in a subject who was in diacerein arm of the study. This adverse event has not been reported in relation to diacerein therapy in previous studies. Several in vitro studies have demonstrated the important role of TNF-α and IL-1α in the constitution of granulomas and immune protection during the early phase of the granulomatous infections like TB. It has been seen that IL-12 also has significantly increased lytic activity against M. tuberculosis-infected cells. Purified NK cells from normal volunteers or from HIV-1-infected subjects were shown in a study to have elevated lytic activity against M. tuberculosis -infected monocytes after IL-2 or IL-12 stimulation. We propose that diacerein apart from IL-1 inhibition might also inhibit other interleukins like IL-12, IL-2 and thereby decreases body’s resistance to tubercle bacilli specially during the first 2 months of infection. Patient developed TB after 2 months of instituting diacerein therapy which also points to the fact that diacerein might have decreased host’s interleukin levels low enough to reactivate TB [42-43]. However, these findings require confirmation via the measurement of IL levels.
The compliance of the patients was tested by counting the empty blister packs and the number of capsules or tablets that remain [44]. Purposefully, they were given few extra tablets/capsules (whose count was maintained by the investigator) than required. This helped in determining the compliance better. The compliance in all the three groups was high (diacerein- 93%, diclofenac- 95% and combination- 96%). Though our study was not a double blinded one, investigators blinded the end-point by blinding the assessor. This type of study is called PROBE study (Prospective, randomized, open-label, blinded-endpoint) and has been shown to be as effective as double blinded studies [45].
The risk-benefit ratio associated with long-term use of NSAIDs and analgesics is well documented in the literature, [46] but clinical studies on the use of NSAIDs for more than 6 weeks in patients with OA are rare [47]. NSAIDs, particularly the selective COX 2 inhibitors, are known to exert a higher risk for thromboembolic disorders such as myocardial infarction or stroke [48]. In this context, it is important to consider that most patients affected by OA also experience disorders, or at least risk factors, of the cardiovascular system and that according to the European Agency for the Evaluation of Medicinal Products [49] and the Food and Drug Administration, [50] NSAIDs should be administered at the lowest possible dose for the shortest period. In contrast, cardiovascular adverse events in patients treated with diacerein can be considered very rare [23]. Present study did not find the incidence of a single cardiovascular related adverse event in diacerein group. Moreover, larger studies that have been continued for 3 years have found that diacerein decreases the progression of joint space narrowing in OA of hip when compared to placebo. Though the short term analgesic potential may be equal to or slightly less than that of NSAIDs, the carry over effect of its analgesic activity and its potential role in delaying the progression of the disease makes it an ideal choice for most OA patients. This drug can be complemented with the use of standard NSAIDs when required. This will diminish the use of NSAIDs as well as the side effects that come along. However, patient education about the disease and the beneficial role of other non-pharmacological therapies must be emphasized. In conclusion, the findings of this study indicate that diacerein is an effective treatment for symptomatic knee OA. In addition, it has a long carryover effect and an acceptable safety profile.
Competing Interests
There was no conflict of interest during and at the end of study.
1931
References
- Pelletier JP, Martel-Pelletier J. Therapeutic targets in osteoarthritis: from today to tomorrow with new imaging technology. Ann Rheum Dis 2003 Nov; 62: 79–82.
- Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum 2001; 44: 1237–47.
- Wood DD, Ihrie EJ, Hamerman D. Release of interleukin-1 from human synovial tissue in vitro. Arthritis Rheum 1985; 28: 853–62.
- Pelletier JP, DiBattista JA, Roughley P, McCollum R, Martel- Pelletier J. Cytokines and inflammation in cartilage degradation. Rheum Dis Clin North Am 1993; 19: 545–68.
- Pelletier JP, Roughley PJ, DiBattista JA, McCollum R, Martel- Pelletier J. Are cytokines involved in osteoarthritic pathophysiology? Semin Arthritis Rheum 1991; 20: 12–25.
- LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, Guilak F. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum 2001; 44: 2078–83.
- Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum 2005; 52:128–35.
- Pavelka K, Trc T, Karpas K, Vitek P, Sedlackova M, Vlasakova V, Bohmova J, Rovensky J. The Efficacy and Safety of Diacerein in the Treatment of Painful Osteoarthritis of the Knee: A randomized, multicenter, double-blind, placebo-controlled study with primary end points at two months after the end of a three-month treatment period. Arthritis Rheum 2007; 56: 4055–64.
- Lequesne M. Symptomatic slow-acting drugs in osteoarthritis: a novel therapeutic concept? Rev Rheum Engl 1994; 61: 69–73.
- Felisaz N, Boumediene K, Ghayor C, Herrouin JF, Bogdanowicz P, Galerra P, Pujol JP. Stimulating effect of diacerein on TGF-beta 1 and beta 2 expression in articular chondrocytes cultured with and without interleukin-1. Osteoarthritis Cartilage 1999; 7: 255–64.
- Smith GN Jr., Myers SL, Brandt KD, Mickler EA, Albrecht ME. Diacerein treatment reduces the severity of osteoarthritis in the canine cruciate-deficiency model of osteoarthritis. Arthritis Rheum 1999; 42: 545–54.
- Douni E, Sfikakis PP, Haralambous S, Fernandes P, Kollias G. Attenuation of inflammatory polyarthritis in TNF transgenic mice by diacerein: comparative analysis with dexamethasone, methotrexate and anti-TNF protocols. Arthritis Res Ther 2004; 6: R65–72.
- Brandt KD, Smith G, Kang SY, Myers S, O’Connor B, Albrecht M. Effects of diacerein in an accelerated canine model of osteoarthritis. Osteoarthritis Cartilage. 1997; 5: 438-49.
- Franchi-Micheli S, Lavacchi L, Friedmann CA, Zilletti L. The influence of rhein on the biosynthesis of prostaglandin-like substances in-vitro. J Pharm Pharmacol 1983; 35: 262–4.
- Petrillo M, Montrone F, Ardizzone S, Caruso I, Bianchi-Porro G. Endoscopic evaluation of diacetylrhein-induced gastric mucosal lesions. Curr Ther Res 1991; 49: 10–15.
- Nguyen M, Dougados M, Berdah L, Amor B. Diacerein in the treatment of osteoarthritis of the hip. Arthritis Rheum 1994; 37: 529–36.
- Lequesne M, Berdah L, Gerentes I. Efficacy and tolerance of diacerein in the treatment of gonarthrosis and coxarthrosis. Rev Prat 1998; 48: S31–35.
- Pelletier JP, Yaron M, Haraoui B, Cohen P, Nahir MA, Choquette D, Wigler I, Rosner IA, Beaulieu AD. Efficacy and safety of diacerein in osteoarthritis of the knee: a double-blind, placebo controlled trial. The Diacerein Study Group. Arthritis Rheum 2000; 43: 2339–48.
- Lingetti M, D’Ambrosio PL, Di Grezia, Sorrentino P, Lingetti E: A controlled study in the treatment of osteoarthritis with diacerein. Curr Ther Res 1982; 31: 408-12.
- Louthrenoo W, Nilganuwong S, Aksaranugraha S, Asavatanabodee P, Saengnipanthkul S. The efficacy, safety and carry-over effect of diacerein in the treatment of painful knee osteoarthritis: a randomised, double-blind, NSAID-controlled study. Osteoarthritis Cartilage 2007; 15: 605-14.
- Pham R, Henanff AL, Ravaud P. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomized controlled study in symptomatic knee osteoarthritis .Ann Rheum Dis 2004; 63: 1611–17.
- Fagnani F, Bouvenot G, Valat JP. Medico-economic analysis of diacerein with or without standard therapy in the treatment of osteoarthritis. Pharmacoeconomics 1998; 13: 135-46.
- Rintelen B, Neumann K, Leeb BF. A meta-analysis of controlled clinical studies with diacerein in the treatment of osteoarthritis. Arch Intern Med 2006; 166: 1899-1906.
- Dougados M, Nguyen M, Berdah L, Mazieres B, Vignon E, Lequesne M, for the ECHODIAH Investigators Study Group. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Arthritis Rheum 2001; 44: 2539–47.
- Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum 1986; 29: 1039–49.
- Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis 1957; 16: 494–502.
- Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988; 15: 1833–40.
- Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop 1989; 248: 13–4.
- Lequesne MG, Mery C, Samson M, Gerard P. Indexes of severity for osteoarthritis of the hip and knee. Validation- value in comparison with other assessment tests. Scandinavian Journal of Rheumatology 1987; 65: 85-9.
- Ware JE, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36) I. Conceptual framework and item selection. Med Care 1992; 30: 473-83.
- Brandt KD. Osteoarthritis. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison’s Principles of Internal Medicine 17ed, Mc Graw Hill, 2008: 2158-65.
- Agarwal K. Osteoarthritis is India’s No. 1 ailment. Times of India (Delhi edition) 2007 Sep 6: 12 (Col 1).
- Hochberg, Marc C. Rheumatology., 3rd Ed. New York: Elsevier Limited, 2003.
- Fidelix TSA, Soares BGDO, Trevisani VF M. Diacerein for osteoarthritis. Cochrane Database of Systematic Reviews 2006, Issue 1. Art. No.: CD005117. DOI: 10.1002/14651858.CD005117, pub2.
- Tang FL, Wu DH, Lu ZG, Huang F, Zhou YX. The efficacy and safety of diacerein in treatment of painful osteoarthritis of the knee. 11º Asia Pacific League of Associations for Rheumatology Congress, Jeju Island, Korea, 11th -15th September, 2004.
- Chantre P, Cappelaere A, Leblan D, Guedon D, Vandermander J, Fournie B. Efficacy and Tolerance of Harpagophytum Procumbens versus Diacerhein in Treatment of Osteoarthritis. Phytomedicine 2000; 7: 177-83.
- Lequesne M, Berdah L, Gerentes I. Efficacy and tolerance of diacerhein in the treatment of gonarthrosis and coxarthrosis. Rev Prat 1998; 48: S31-5.
- Zheng WJ, Tang F, Li J, Zhang FC, Li ZG, Su Y, Wu DH, Ma L, Zhou HQ, Huang F, Zhang JL, Liang DF, Zhou YX, Xu H. Evaluation of efficacy and safety of diacerein in knee osteoarthritis in Chinese patients. Chin Med Sci J 2006; 21: 75-80.
- Rabenda V, Manette C, Lemmens R, Mariani AM. Prevalence and impact of osteoarthritis and osteoporosis on health-related quality of life among active subjects. Aging Clin Exp Res 2007; 19: 55-60.
- Yagi T, Miyawaki Y, Nishikawa T, Yamauchi K, Kuwano S. Involvement of prostaglandin E-like material in the purgative action of rhein anthrone, the intraluminal active metabolite of sennosides A and B in mice. J Pharm Pharmacol 1988; 40: 27–30.
- Isoda K, Sawada S, Ayaori M, Matsuki T, Horai R, Kagata Y, Miyazaki K, Kusuhara M, Okazaki M, Matsubara O, Iwakura Y, Ohsuzu F. Defi ciency of interleukin-1 receptor antagonist deteriorates fatty liver and cholesterol metabolism in hypercholesterolemic mice. J Biol Chem 2005; 280: 7002-9.
- Hernandez-Pando R, Orozco H, Arriaga K, Sampieri A, Larriva-Sahd J, Madrid-Marina V. Analysis of the local kinetics and localization of interleukin-1α, tumour necrosis factor-α and transforming growth factor-β, during the course of experimental pulmonary tuberculosis. Immunology 1997; 90: 607-7.
- Denis M. Interleukin-12 (IL-12) augments cytolytic activity of natural killer cells toward Mycobacterium tuberculosis-infected human monocytes. Cell Immunol 1994; 156: 529-36.
- Steiner JF, Earnest MA. The language of medication-taking. Ann Intern Med 2000; 132: 926-30.
- Smith DH, Neutel JM, Lacourcière Y, Kempthorne-Rawson J. Prospective, randomized, open-label, blinded-endpoint (PROBE) designed trials yield the same results as double-blind, placebo- controlled trials with respect to ABPM measurements. J Hypertension 2003; 21: 1291-8.
- Brandt KD. Should osteoarthritis be treated with nonsteroidal anti- infl ammatory drugs? Rheum Dis Clin North Am 1993; 19: 697-712.
- Dieppe P, Cushnaghan J, Jasani MK, McCrae F, Watt IA. Two-year, placebo controlled rial of non-steroidal anti-infl ammatory therapy in osteoarthritis of the knee joint. Br J Rheumatol 1993; 32: 595-600
- Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet 2004; 364: 2021-9.
- The European Agency for the Evaluation of Medicinal Products. Questions and answers on non-selective NSAIDs: CHMP review of safety of non-selective NSAIDs. London, England: European Agency for the Evaluation of Medicinal Products; 2005. Publication EMEA/250423/2005.
- Food and Drug Administration. Public health advisory non-steroidal anti-infl ammatory drug products (NSAIDs). 2006. https://www.fda.gov/ cder/drug /advisory/nsaids.htm. accessed February 1, 2007.












