Keywords
Degenerative spondylolisthesis; Neurogenic claudication; Balloon neuroplasty
Introduction
Degenerative spondylolisthesis (DS) is the most common orthopedic condition affecting the spine condition. DS is the slipping forward of a single lumbar vertebra on another, with an intact neural arch. Grade 1 represent a slip of <25% of the vertebral body, grade 2 represents a slip of between 25% and 50%, grade 3 represents a slip of between 50% and 75%, and grade 4 represents a slip of < 75% vertebral body being slipped forward. Grade 1 or 2 accounts for the majority of DS cases. Slippage most commonly occurs at the L4-L5 level and rarely exceeds 30% of the vertebral width [1].
DS results in spinal stenosis and clinical presentation of neurogenic claudication, or low back pain. Claudication is a usually referring to impairment in walking, or to pain, discomfort, numbness, or tiredness in the legs that occurs during walking or standing and is relieved by rest.
Generally, surgical treatment is better than nonsurgical treatment [2,3]. Lumbar spinal stenosis (LSS) can be defined as a narrowing of the spinal canal caused by both bone and soft tissues, which lead to mechanical compression of the spinal nerve roots. The compression of these nerve roots can present with sign and symptoms, including weakness, reflex changes in reflexs, gait disturbances, bowel or bladder dysfunction, motor and sensory changes, radicular pain or atypical leg pain, and neurogenic claudication [4,5].
The treatment for DS vary, and include medication, exercise, steroid injections, and surgery [6,7]. Lumbar epidural steroid injection is commonly used to treat patients with LSS [5,8-10]. Also, Percutaneous epidural adhesiolysis, which interventional pain management technique, has been used for those patients with refractory chronic low back pain or following failed back surgery syndrome [11-14]. The goal of adhesiolysis is to ameliorate aberrant adhesion and to deliver medication to the targeted site [15].
Balloon neuroplasty (BNP) is additional treatment technique [16,17] in which intermittent ballooning is used to produce distension of the epidural space, increased mechanical detachment of a perineural adhesions, and relieve [16]. Previous studies have reported that BNP to be effective in the treatment of lumbar stenosis [16,17] but have shown that there is no correlation of spinal canal dimensions with the efficacy of BNP in spinal stenosis [16].
In generally, the presence of DS is unsuccessful outcome in treated with PEN [18,19]. This may be because DS is associated with increased segmental instability and decreased cross area of spinal diameter [7]. Moreover, Choi et al. [16] have no significant pain relief following BNP, regardless of whether DS was present or not. This study did not investigate the effect of BNP depending on DS grade.
To our knowledge, there have thus far been no published studied on efficacy of BNP according to varying grades of DS. Our study was conducted to assess the therapeutic efficacy of BNP according to DS grade, evaluating the short-term results of the treatment.
Methods
Study design
A total of 107 patients between 18 and 80 years, of age were enrolled in this retrospective study. Gender was described in Table 1. The diagnosis of either grade 1 DS (n=56), or grade 2 DS (n=51) was made based on clinical symptoms, neurological assessment, and imaging studies that included plain radiography as well as magnetic resonance imaging (MRI) of the lumbar spine. We obtained approval from IRB and informed consent was obtained from the participants.
| N=107 |
DS 1 (n=56) |
DS 2 (n=51) |
| Age (yrs) |
68.2 (10.6) |
74.3 (9.0) |
| Gender (M : F) |
20:36 |
18:33 |
| Pain Duration (yrs) |
3.4 (2.9) |
2.5 (2.7) |
| Pain level |
|
-- |
| L2-L3 |
3 |
1 |
| L3-L4 |
5 |
3 |
| L4-L5 |
32 |
28 |
| L5-S1 |
8 |
10 |
| L2-L3 + L3-L4 |
1 |
-- |
| L3-L4 + L4-L5 |
4 |
5 |
| L4-L5 + L5-S1 |
3 |
4 |
Table 1: Demographic data.
The study inclusion criteria for patients with DS were follow (Figures 1A and 1B); (1) a diagnosis of degenerative spondylolisthesis with back or leg pain; (2) clear evidence of DS on MRI or simple radiographic images; (3) grade 1 or 2 spondylolisthesis [20]. The exclusion criteria were as follows; (1) unclear description of symptoms; (2) previous back surgery; (3) grade 3 or 4 spondylolisthesis; (4) mild- or moderate-grade spinal stenosis; (5) presence of serious neurologic deficit.
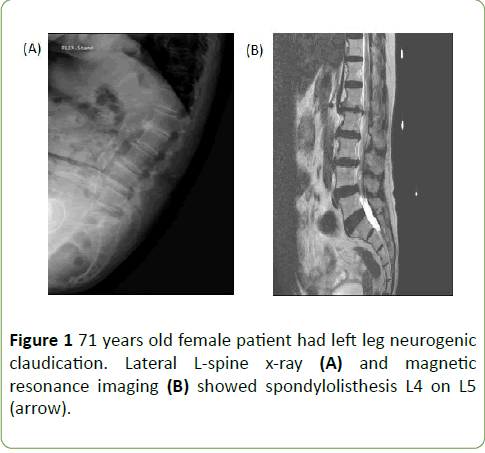
Figure 1: 71 years old female patient had left leg neurogenic claudication. Lateral L-spine x-ray (A) and magnetic resonance imaging (B) showed spondylolisthesis L4 on L5 (arrow).
The BNP procedures were performed in the operating room. With the patient in the prone position, the needle insertion site was prepared with betadine and draped. A 10 G guide needle, was inserted under fluoroscopic guidance into the caudal epidural space through the sacral hiatus. Once the needle was confirmed to be in the caudal epidural space, a 3-5 ml of contrast agent (Omnipaque® 300) was used to obtain a lumbar epidurogram in order to identify filling defect. A balloon catheter was advanced through the needle to the area of the filling defect or the site of pathology, as determined by MRI. Following the satisfactory positioning of the catheter, BNP was conducted by performing gentle side-to-side movement of the catheter with ballooning. The balloon was filled with 0.13 ml of contrast agent, and each ballooning process was limited to 5 seconds (Figure 2). After the procedure, if contrast agent in the anterior epidural space spread was observed to upward above the level of the affected site, successful adhesiolysis was assumed to have been achieved. A 6 ml of a mixture of 0.5% lidocaine, 5 mg dexamethasone, and 1,500 IU hyaluronidase was administered via the catheter. No complications such as bleeding or damage to the dura during procedure.
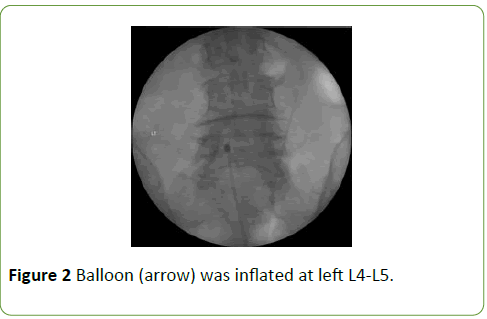
Figure 2: Balloon (arrow) was inflated at left L4-L5.
Subjects’ responses to the procedure were assessed using visual analogue scale score (VAS) and the Oswestry disability index (ODI). The assessments were performed at 2 weeks and 6 months after BNP. VAS differences were evaluated by the Chi-squared test. Correlations between pain relief and pain duration, age, and gender were evaluated by the Spearman rank correlation test. P-value<0.05 were considered significant. Statistical analysis was performed by standard software (SPSS v23; SPSS Inc [IBM], Chicago, IL, USA.
Results
The 107 patients were divided into two groups studied according to the grading at diagnosis (DS 1vs DS 2). A summary of patient characteristics, including herniation levels, and location of pain is provided in Table 1. In both patient groups, L4-L5 was most frequently involved. In the DS 1 group, mean VAS was 7.8, 5.5 and 5.5 at pre-treatment, 2 weeks post treatment, and 6 months post treatment, respectively in DS 1 group. In DS 2, mean VAS was 7.7, 5.3 and 5,4 at pretreatment, 2 weeks post treatment, and post 6 months post treatment, respectively.
The mean post-treatment VAS score, and ODI at 2 weeks and 6 months were not statistically significant change compared with pre-treatment values in each VAS in each group (Tables 2 and 3). In addition, VAS difference between two groups were not significant (Table 4). Among the DS 1 patients, 37% of patients had VAS > 50% at both at the 2 weeks and 6 months follow up. Among the patients with DS 2 group, 17.9% and 21% of patients had VAS values > 50% at 2 weeks and 6 months follow up assessment, respectively (Table 4).
| Variables |
DS 1 (n=56) |
DS 2 (n=51) |
P value |
| Pre VAS |
7.8 (0.7) |
7.7 (0.6) |
0.62 |
| Post 2 weeks |
5.5 (2.2) |
5.3 (1.4) |
0.74 |
| Post 6 months |
5.5 (2.1) |
5.4 (1.6) |
0.8 |
Values are mean (SD)
Table 2: Change of Visual Analogue Scale (VAS) score.
| ODI |
DS 1 (n=56) |
DS 2 (n=51) |
| Pre-treatment |
54.7 (5.6) |
57.7 (3.9) |
| Post 2 weeks |
53.2 (4.3) |
54..6 (6.2) |
| Post 6 months |
53.8 (5.7) |
53.8 (3.6) |
Values are mean (SD)
Table 3: Change of Oswetry disability index (ODI).
| VAS |
DS 1 (n=56) |
DS 2 (n=51) |
| 2 weeks |
6 months |
2 weeks |
6 months |
| 0-10% |
16 (31.4%) |
13 (25.5%) |
12 (21%) |
12 (21%) |
| 11-20% |
3 (5.9%) |
8 (15.7%) |
6 (10.7%) |
6 (10.7%) |
| 21-30% |
4 (7.8%) |
8 (15.7%) |
10 (17.9%) |
10 (17.9%) |
| 31-40% |
5 (9.8%) |
0 |
12 (21.4%) |
10 (17.7%) |
| 41-49% |
4 (7.8%) |
4 (7.8%) |
6 (10.7%) |
6 (10.7%) |
| 50% ≤ |
19 (37.3%) |
19 (37.3%) |
10 (17.9%) |
12 (21%) |
Table 4: The number, and proportion of patients who achived the percentage range improvement of pain.
In DS 1 group, there was significant correlation between pain duration and at 2 weeks VAS (Table 5). Three patients with DS 1 and with DS 2 had done surgery after BNP (Table 6).
| Variables |
DS 1 (n=56) |
DS 2 (n=51) |
| Pre VAS |
2 weeks |
6 month |
Pre VAS |
2 weeks |
6 month |
| Pain Duration |
Coefficient |
0.36 |
0.53 |
0.1 |
0.14 |
-0.12 |
0.08 |
| P value |
0.11 |
0.01 |
0.11 |
0.43 |
0.49 |
0.04 |
| Age |
Coefficient |
0.32 |
-0.21 |
0.02 |
0.11 |
-0.22 |
-0.27 |
| P value |
0.15 |
0.37 |
0.92 |
0.55 |
0.22 |
0.13 |
| Gender |
Coefficient |
-0.08 |
-0.61 |
-0.51 |
-0.05 |
0.26 |
-0.07 |
| P value |
0.74 |
0 |
0.02 |
0.77 |
0.88 |
0.69 |
Table 5: Correlation between pain relief and pain duration, age, gender at 2 weeks and 6 months follow-up.
| Variables |
DS 1 (n=56) |
DS 2 (n=51) |
| Surgery |
3 |
3 |
| Additional procedure |
2 |
0 |
| Operation recommend |
2 |
2 |
Table 6: Number of patients undergoing surgery.
Discussion
This retrospective observational study showed that BNP did not provided sufficient pain relief for patients with grade 1 or 2 DS. These findings are inconsistent with those of previous reports. Previous studied found that LSS patients obtained significant pain relief and functional improvements over a 12 months period after BNP [16,21]. The authors postulated that distension of the epidural space by intermittent ballooning can lead to increased detachment of a perineural adhesion, increased space at the stenotic area, or reduced venous congestion or stasis [16,21].
By contrast, our study did not observe significant pain relief. Percutaneous epidural adhesiolysis, or BNP have been reported to be an effective method in the treatment of degenerative LSS [11,15-17,21,22]. DS is typically manifested as a dynamic mechanical compression of the dura and nerve root sheaths of the cauda equina. The compression can result in hyperemia, venous congestion, and nerve root edema [7]. The pain relief achieved by BNP might be result of the gave the dissolution of the aberrant adhesions, as well as the targeted delivery of medication into the affected site [23]. However, in DS, neural structures are directly compressed by stenosis. Also, DS lead to segmental instability and diminished cross-sectional area of spinal canal, apparent thickening and buckling of the ligamentum flavum, or hypertrophy of adjacent facet joints [7]. Moreover, the pathophysiology of these conditions is complicated, but not the only factor involved [24]. Additional, complicating factors, which include the presence of multiple sites of compression and/or the cephalad/caudad extension of the compression might be involved whether or not spinal stenosis is symptomatic [25]. Another factor underlying the inconsistency between symptoms and the degree of spinal canal stenosis is the use of static images to determine the dimensions of the of spinal canal in what is dynamic condition [26]. In our study therefore, BNP was found not to be effective treatment for DS. However, a previous study found no correlation with whether DS or not after BNP in pain relief, instead the absence of diabetes was higher in the successful responders than in the non-responders [16].
In present study, patient with long-term pain achieved less pain relief in DS 1 group (at 2 weeks follow up). However, at 6 months, there was no correlation pain relief and pain duration. Moreover, another study has reported no association with pain duration and pain reduction [27].
Conclusion
This study has several limitations. The outcomes of the procedure outcome were assessed by using the patient’s pain score; there were assessments of functional outcome measurement or measurement, in reduction in medication, in the proportions of patients with 50% pain relief, and in improvement in disability status. Further studies of patients with multiple level LSS or grade 3, 4 DS are therefore warranted for the evaluating of BNP for the treatment of DS.
In conclusion, at the 6-month follow-up period, BNP was found not be a suitable treatment modality for patients with grade 1 or grade 2 DS.
22471
References
- Jacobsen S, Sonne-Holm S, Rovsing H, Monrad H, Gebuhr P (2007) Degenerative lumbar spondylolisthesis: an epidemiological perspective: the Copenhagen Osteoarthritis Study. Spine (Phila Pa 1976) 32: 120-125.
- Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, et al. (2007) Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med 356: 2257-2270.
- Rihn JA, Hilibrand AS, Zhao W, Lurie JD, Vaccaro AR, et al. (2015) Effectiveness of surgery for lumbar stenosis and degenerative spondylolisthesis in the octogenarian population: analysis of the Spine Patient Outcomes Research Trial (SPORT) data. J Bone Joint Surg Am 97: 177-185.
- Botwin KP, Gruber RD (2003) Lumbar spinal stenosis: anatomy and pathogenesis. Phys Med Rehabil Clin N Am 14: 1-15.
- Simotas AC, Dorey FJ, Hansraj KK, Cammisa F Jr. (2000) Nonoperative treatment for lumbar spinal stenosis. Clinical and outcome results and a 3-year survivorship analysis. Spine (Phila Pa 1976) 25: 197-203; 203-194.
- Daffner SD, Wang JC (2009) The pathophysiology and nonsurgical treatment of lumbar spinal stenosis. Instr Course Lect 58: 657-668.
- Koreckij TD, Fischgrund JS (2015) Degenerative spondylolisthesis. J Spinal Disord Tech 28: 236-241.
- Koc Z, Ozcakir S, Sivrioglu K, Gurbet A, Kucukoglu S (2009) Effectiveness of physical therapy and epidural steroid injections in lumbar spinal stenosis. Spine (Phila Pa 1976) 34: 985-989.
- Delport EG, Cucuzzella AR, Marley JK, Pruitt CM, Fisher JR (2004) Treatment of lumbar spinal stenosis with epidural steroid injections: a retrospective outcome study. Arch Phys Med Rehabil 85: 479-484.
- Harrast MA (2008) Epidural steroid injections for lumbar spinal stenosis. Curr Rev Musculoskelet Med 1: 32-38.
- Manchikanti L, Pampati V, Fellows B, Rivera JJ, Damron KS, et al. (2001) Effectiveness of percutaneous adhesiolysis with hypertonic saline neurolysis in refractory spinal stenosis. Pain Physician 4: 366-373.
- Epter RS, Helm S, Hayek SM, Benyamin RM, Smith HS, et al. (2009) Systematic review of percutaneous adhesiolysis and management of chronic low back pain in post lumbar surgery syndrome. Pain Physician 12: 361-378.
- Park CH, Lee SH, Jung JY (2011) Dural sac cross-sectional area does not correlate with efficacy of percutaneous adhesiolysis in single level lumbar spinal stenosis. Pain Physician 14: 377-382.
- Park CH, Lee SH (2013) Effectiveness of percutaneous transforaminal adhesiolysis in patients with lumbar neuroforaminal spinal stenosis. Pain Physician 16: E37-43.
- Manchikanti L, Cash KA, McManus CD, Pampati V, Singh V, et al. (2009) The preliminary results of a comparative effectiveness evaluation of adhesiolysis and caudal epidural injections in managing chronic low back pain secondary to spinal stenosis: a randomized, equivalence controlled trial. Pain Physician 12: E341-354.
- Choi SS, Lee JH, Kim D, Kim HK, Lee S, et al. (2016) Effectiveness and factors associated with epidural decompression and adhesiolysis using a balloon-inflatable catheter in chronic lumbar spinal stenosis: 1-year follow-up. Pain Med 17: 476-487.
- Kim SH, Koh WU, Park SJ, Choi WJ, Suh JH, et al. (2012) Clinical experiences of transforaminal balloon decompression for patients with spinal stenosis. Korean J Pain 25: 55-59.
- Lee JH, Lee SH (2013) Clinical effectiveness of percutaneous adhesiolysis and predictive factors of treatment efficacy in patients with lumbosacral spinal stenosis. Pain Med 14: 1497-1504.
- Moon DE, Park HJ, Kim YH (2015) Assessment of clinical outcomes of cervical epidural neuroplasty using a Racz-catheter and predictive factors of efficacy in patients with cervical spinal pain. Pain Physician 18: E163-170.
- Kim SH, Choi WJ, Suh JH, Jeon SR, Hwang CJ, et al. (2013) Effects of transforaminal balloon treatment in patients with lumbar foraminal stenosis: a randomized, controlled, double-blind trial. Pain Physician 16: 213-224.
- Manchikanti L, Saini B, Singh V (2001) Spinal endoscopy and lysis of epidural adhesions in the management of chronic low back pain. Pain Physician 4: 240-265.
- Boswell MV, Trescot AM, Datta S, Schultz DM, Hansen HC, et al. (2007) Interventional techniques: evidence-based practice guidelines in the management of chronic spinal pain. Pain Physician 10: 7-111.
- Arbit E, Pannullo S (2001) Lumbar stenosis: A clinical review. Clin Orthop Relat Res 384: 137-143.
- Campbell MJ, Carreon LY, Glassman SD, McGinnis MD, El-Mlinger BS (2007) Correlation of spinal canal dimensions to efficacy of epidural steroid injection in spinal stenosis. J Spinal Disord Tech 20: 168-171.
- Sirvanci M, Bhatia M, Ganiyusufoglu KA, Duran C, Tezer M, et al. (2008) Degenerative lumbar spinal stenosis: correlation with Oswestry Disability Index and MR imaging. Eur Spine J 17: 679-685.
- Radcliff KE, Rihn J, Hilibrand A, DiIorio T, Tosteson T, et al. (2011) Does the duration of symptoms in patients with spinal stenosis and degenerative spondylolisthesis affect outcomes?: analysis of the Spine Outcomes Research Trial. Spine (Phila Pa 1976) 36: 2197-2210.







