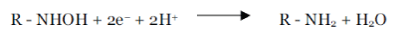Key words
|
| |
| Clonazepam, Glassy carbon electrode, Cyclic Voltammetry, Galvanostatic technique |
| |
Introduction
|
| |
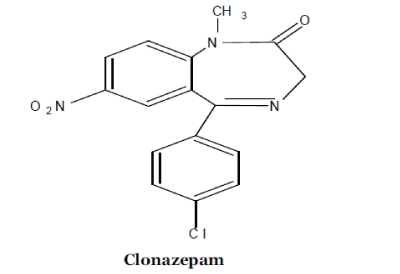
| Clonazepam is a drug, which is a benzodiazepine derivative. It is highly potent anticonvulsant and anxiolytic. It is a chlorinated derivative of nitrazepam and a nitrobenzodiazepine like nitrazepam [1-6]. Clonazepam’s primary mechanism of action is via modulating GABA (gamma-aminobutyric acid) function in the brain, via the benzodiazepine receptor, which in turn leads to enhanced GABAergic inhibition of neuronal firing. In addition Clonazepam decreases the utilization of 5- HT (serotonin) by neurons and has been shown to bind tightly to central type benzodiazepine receptors, because of its strong anxiolytic and anticonvulsant properties. It is said to be among the class of “highly potent” benzodiazepines. The anticonvulsant properties of benzodiazepine due to inhibition of sustained high frequency starts repeated firing. Benzodiazepines, including clonazepams, bind to galial cell membranes with high affinity. Clonazepam decreases levels of acetylcholine and release of prolactin. Benzodiazepine inhibits cold-induced thyroid stimulating hormone (also known as TSH or thyrotropin) release. Benzodiazepines act via micro molar benzodiazepine binding sites as Ca+2 channel blockers and significantly inhibit depolarization- nitro group and we have tried to reduce its nitro group and monitor it through voltammetric analysis. Voltammetric techniques showed themselves to be an mechanism and analytical determination of drugs, excellent alternative for the study of the reaction sensitive calcium uptake [7-10]. Clonazepam contains especially heterocyclic nitro compounds [11-13]. Compared with other methods developed for Clonazepam determination, this new procedure (Cyclic Voltammetry) possesses following advantages, such as a low detection limit, a rapid response, excellent reproducibility, simplicity and low cost [14]. The electrochemical behavior of Clonazepam was studied by cyclic voltammetric techniques on glassy carbon electrode. Typical cyclic voltammograms for Clonazepam recorded within the wide range (1000 to –1000 mV) of the potential at different scan rate. The shape of cyclic voltammograms clearly indicated the reversible nature of reduction. |
| |
 |
| |
|
Scheme for Chemical Reaction:-
|
| |
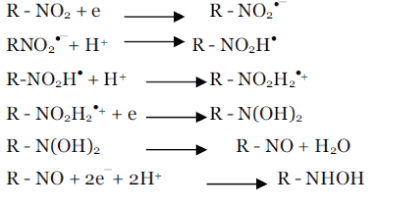
| Electrochemical reduction of nitro compounds strongly depends on the pH of medium. General mechanism of reduction of nitro compounds in different media can be given as- |
| |
| (i) in acidic medium (under high proton availability situation ) |
| |
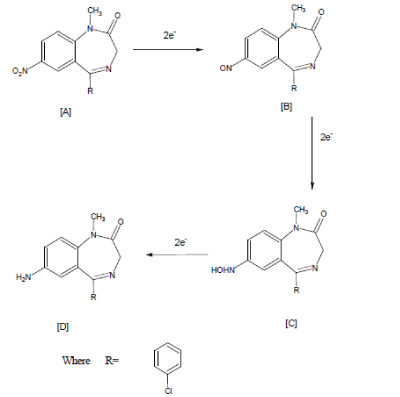
| The reduction of the benzodiazepine is a complex process, involving six electrons for complete reduction of the nitro group to the amine derivative [15-17] |
| |
 |
| |
| Under anaerobic conditions or low oxygen pressure, the reduction process is similar to that of observed for nitrobenzene. The reduction mechanism for several aromatic and heterocyclic nitro compounds was presented by Zuman and Coworkers [18-26]. A total of two electrons and two protons is involved in the formation of the nitroso (R-NO) intermediate, two more electrons and protons result in the hydroxylamine (R-NHOH) [27]. |
| |
 |
| |
| The addition of two more electrons results in the formation of the amine. |
| |
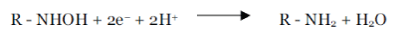 |
| |
| In neutral and basic medium (Under the condition of low proton availability) |
| |
 |
| |
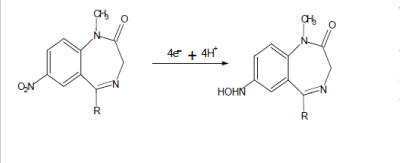
| Generally the E1/2 values for the nitro group reduction change with the lateral chain at the N1 position of the heterocyclic ring [29-31]. Some compounds showed only one reduction wave involving six electrons in acidic medium. |
| |
| The possibilities of the formation of the amino derivative and hydroxylamine derivative of benzodiazepine were explored whenever the reaction performed under acidic, neutral and basic medium respectively. Six and four-electrons change occurs with the formation of corresponding product in acidic and basic medium respectively. Present work describes cathodic behavior of Clonazepam in acidic medium using galvanostatic technique. |
| |
Material and Methods
|
| |
| A stock standard solution of Clonazepam in bulk (1×10-3 mol L-1) was prepared in Di-methyl formamide (DMF) then stored at 4°C until assay. A series BR buffer of pH values 2 to 11 was prepared and used as a supporting electrolyte. 10.0 mL of the BR buffer of pH 7 containing the appropriate concentration of the bulk Clonazepam were transferred into the electrochemical cell, through which a pure nitrogen stream was passed for 10 min for remove the oxygen gas before measurements. Voltammograms were recorded. Quantification of Clonazepam was performed by means of both calibration curve and standard addition methods. The reversibility of the reduction process was investigated by cyclic voltammetric technique. |
| |
| All the solutions were prepared in conductivity water. Electrolytic cell used was a 250ml beaker (tall form) with the provisions of porous pot, magnetic stirrer, magnet bar, thermometer and cathode to measure the cathodic potential vs. saturated calomel electrode SCE). Other components of the cell assembly were as same as followed earlier.[28] |
| |
| Voltammetric measurements were performed with a Metrohm Computrace Voltammetric Analyzer μ AUTOLAB TYPE III Potentiostat Ecochemie (Utrecht, The Netherlands) Model 757 VA. A conventional three-electrode system was used consisting of an Ag/AgCl/KCl reference electrode, a glassy carbon electrode as a working electrode and a graphite rod as auxiliary electrode. The whole measurements were automated and controlled through the programming capacity of the apparatus. The data were treated through a PC connected to the Electrochemical Analyzer version-757 VA computer. |
| |
| A digital pH-meter (CHINO- DB-1011) was used for measuring the pH values of the investigated solutions. The digital pH meter was fitted with a glass electrode and a saturated calomel electrode as the reference, which was previously standardized with buffers of known pH. |
| |
| Concentration of Catholyte prepared ranges from 0.001 M to 0.004 M in 100ml mixture of aqueous 1%(v/v) sulphuric acid and methanol in 3:1(v:v) ratio solution. Magnetic stirrer was used for agitation. The desired current, according to current density range, was applied from the current regulated power supply (galvanostatic), developed by CDPE (Centre for Development of Physics Education), University of Rajasthan, Jaipur. The cathodic potential was measured by digital multimeter RISH Multi 14S via saturated calomel electrode (SCE) through agar-agar Gel Bridge in each case, i.e. at all electrode under investigation in the presence and absence of depolarizer to get suitable current density range for reduction under the experimental conditions imposed. In all cases, a theoretical quantity of current was passed, depending upon the amount of depolarizer taken. |
| |
| The compound from mixture is purified by, passing through the column of Silica Gel. The different constituent gets adsorbed to the different extent, which was then analyzed by the usual various physicochemical methods. |
| |
| Analysis by Physicochemical Method |
| |
| Solid was soluble in ether, alcohol, acetone and partially in water, and burns with sooty flames, showing aromatic nature of compound. |
| |
| A single clear spot on silica gel G plate was obtained in iodine chamber (80% C6H6 + 20% ethyl acetate medium) confirming, that the product was a single compound and not a mixture. |
| |
| IR spectra were recorded in KBr on a SHIMADZU 400-50 |
| |
| Infrared Spectrophotometer (Vmax in cm.-1) |
| |
| 1H-NMR spectra were recorded on JEOL AL300 1HNMR Spectrophotometer using CDCl3 as solvent and TMS as an internal standard (Chemical shift in d ppm) |
| |
|
IR Spectra
|
| |
| IR spectrum of reduced product of clonazepam 2- amino-9-(4-chlorophenyl)-5,7,8,9-tetrahydro-6Hbenzo-( 7) annulene-6-one compound with methane (1:1) showed a sharp one peak for (-NH2) at 3350 cm- 1, i.e. main characteristic peak of NH2 group which shows nitro group completely reduced in amino group. |
| |
| Aromatic C=C streching peak at 1605 cm-1 , aromatic C-H streching at 3080 cm-1 >C=O streching at 1680 cm-1, it means carbonyl group of lactam ring remain intact during reduction of clonazepam. |
| |
|
NMR-Spectra
|
| |
| A singlet appeared at d 4.4 ppm due to (-N-CH2- C=O) group. |
| |
| 1H NMR of –NH2 exhibited broad singlet at d 4.9 ppm. |
| |
| 1H NMR of –N (CH3)-C=O exhibited a broad singlet at δ 7.9 ppm. |
| |
| A multiplet appeared at δ 7.2-8.0 ppm due to aromatic proton of ring. |
| |
RESULT AND DISCUSSION
|
| |
|
A. Potentiostatic Technique:
|
| |
|
B. Cyclic Voltammetric Studies
|
| |
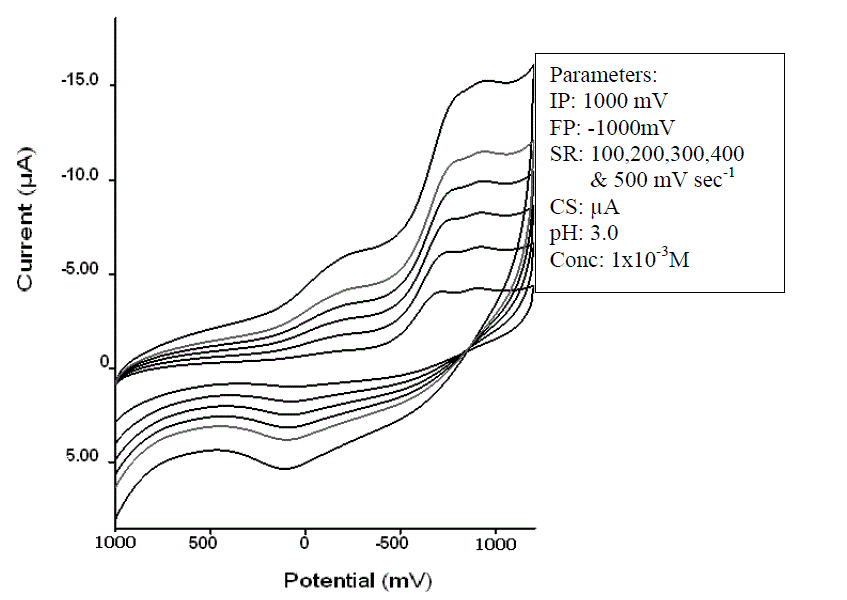
| The effect of the scan rate (n) on the cathodic peak current had been studied by using the same solution and recording CV's at 100, 200,300,400 and 500mV sec-1 scan rate. The effect of scan rate on reduction potential and reversible or irreversible nature of reduction process was also studied by cyclic voltammetry. |
| |
| Typical cyclic voltammograms for Clonazepam recorded within the wide range (1000 to –1000 mV) of the potential at different scan rate. The shape of cyclic voltammograms clearly indicated the reversible nature of reduction. The electrochemical behavior of Clonazepam was studied by cyclic voltammetric techniques on glassy carbon electrode. Clonazepam gave one, well defined reduction peak at -700 mV in the nonaqueous solution which is attributed to the reduction of Clonazepam at glassy carbon electrode. One peak observed in anodic direction of the reverse scans suggesting the reversible nature of the electrode process. The peak potential shifted towards more negative values with increase in scans rate, (Fig: I) confirming the reduction become difficult as the scan rates increases. |
| |
| The effect of scan rate (υ1/2) on stripping peak current (ip) was examined under the above experimental conditions. As the sweep rate is increased from 100 to 500 mV/s at a fixed concentration (1x 10-3 M) of Clonazepam ; (i) the peak potential shifted cathodically and (ii) the peak current increased steadily. |
| |
|
Effect of Scan Rate
|
| |
| The effect of scan rate (n) on the cathodic peak current by using the same solution and recording CV’s at 100,200,300,400 and 500 mVsec-1 scan rate. The effect of scan rate for Clonazepam on the appearance of the cyclic voltammograms can be described by the Randles – Servick equation, cathodic peak current increases as n½. A plot of this equation (ipc/n½) for Clonazepam yields a straight line.On the basis of above studies it is observed that reduction of Clonazepam is possible by voltammetric methods. Linear nature of ip / n½ clearly indicates that reduction of Clonazepam is diffusion controlled and a single cathodic peak is observed. Peak potential shifts towards negative side with increasing scan rates, it shows, at higher scan rate reduction is difficult. Shape of cyclic voltamograms indicates the reversibility of reduction process. Hence on the basis of above study it can be conclude that Clonazepam can be reduced electrochemically. |
| |
|
C. Galvanostatic Technique:
|
| |
|
Polarization Curves
|
| |
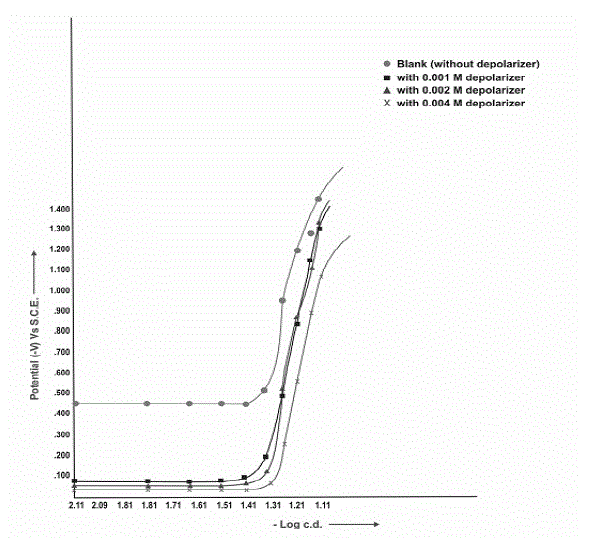
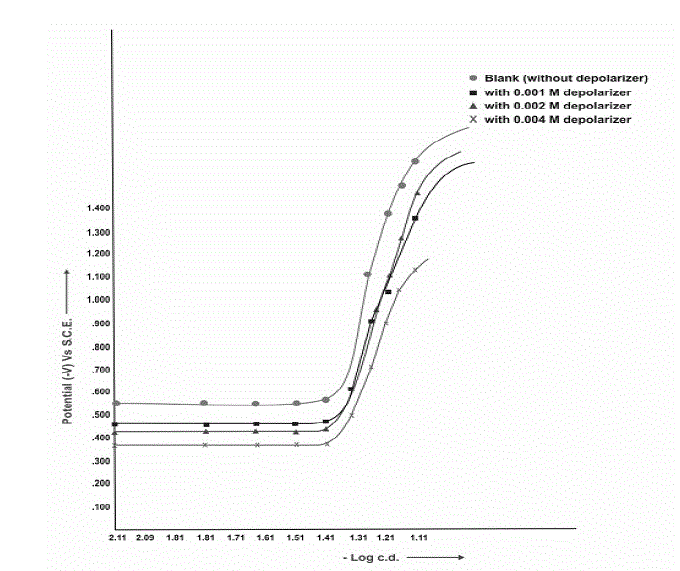
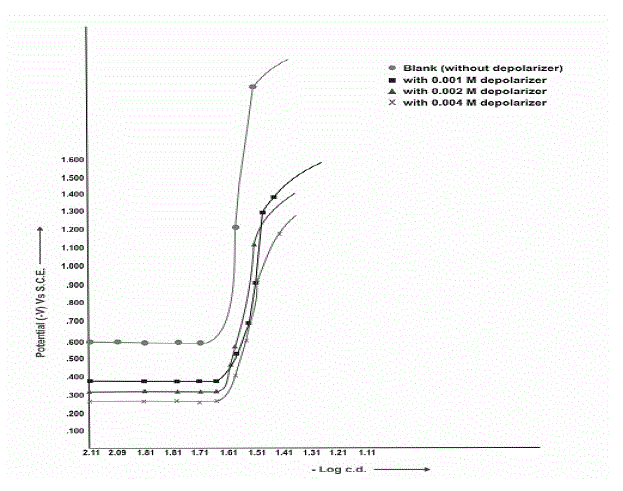
| Polarization curves for the reduction of Clonazepam at Pb, Pb-Hg and Cu-Hg electrodes show that, since, polarization is taking place, thus, reaction may be expected. |
| |
| This has been further confirmed by actual electrolysis of Clonazepam at three (Pb, Pb-Hg, Cu-Hg) electrodes where reductive product can be isolated after passing current for desired duration of time according to Faraday’s law. Fig: II, III and IV show the cathodic polarization curves of clonazepam in 1% (v/v) H2SO4, temperature (303±1K) at Pb, Pb-Hg, Cu-Hg electrodes, respectively, and the proper current density is 0.070, 0.063 and 0.070 Amp.cm-2, respectively, for these electrodes |
| |
| From the available range of current densities (c.d), different current densities were chosen, and the experiments were conducted at each c.d., for all the electrodes investigated (Table-7). It shows that higher ranges of current density are favourable for high yields and the optimum current densities for Pb, Pb-Hg, and Cu-Hg electrodes are 0.070, 0.063 and 0.070 amp.cm-2, respectively. |
| |
|
Effect of Depolarizer Concentration
|
| |
| The concentration of depolarizer was varied from 0.001 M to 0.004M. It was observed from the results that the depolarizer concentration 0.002 M for Pb- Hg electrode gives maximum yield in 1% (v/v) H2SO4 medium (Table 6) |
| |
CONCLUSION
|
| |
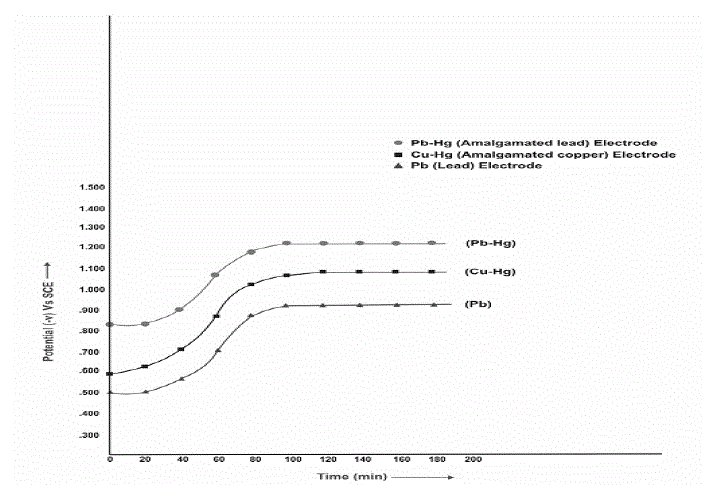
| The formation of, 2-amino-9-(4-chlorophenyl)- 5,7,8,9-tetrahydro-6H-benzo-(7) annulene-6-one compound by the electro reduction of Clonazepam , involves 6 electrons process. (Scheme: 1).This was confirmed by working electrode potential vs. time curves (Fig: V). It is inferred from the curves that, the cathodic potential becomes constant after theoretical time during long-term electrolysis, which is required for, approximately, 6-electron process, according to Faraday’s Law. An electrolytic method for its preparations will be of industrial importance. It is concluded from the results that product can be conventionally prepared by the cathodic reduction of Clonazepam at either of the electrodes investigated. |
| |
AKNOWLEDGEMENT
|
| |
| Authors are thankful to University Grants Commission (UGC), New Delhi for providing financial assistance in the form of a major Research project. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
| `
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |