Keywords
Thoracic aorta; Aneurysm; Endovascular repair
Introduction
Aneurysms of the descending thoracic aorta are life threatening situations, with an estimated incidence of 6-10, 4 cases per 1,00,000 person–years and the incidence seems to increase over time [1,2]. The risk of rupture in patients with untreated descending aortic aneurysms ranges from 46–74%, with five year survival rates ranging from 9–13% [1]. The median diameter of the descending aorta aneurysms at the time of rupture is about 7.2 cm (3) and the mean rate of growth of untreated aneurysms is estimated to be 0.21-0.42 cm/y [3-5].
Treatment entails either conventional surgical treatment by means of a left lateral thoracotomy, where the aneurysm is excised and replaced by a tubular graft, with implantation of the lower intercostal arteries, or the minimally invasive endovascular treatment (TEVAR), performed most commonly by exposing either of the femoral arteries and inserting a stent graft under fluoroscopic guidance. TEVAR was initially developed to treat patients who were considered to not be surgical candidates but is now considered a suitable alternative to open surgery in most cases [6,7].
Surgery is indicated when the size of the descending aorta is over 5.5 cm, or there is rapid expansion of the aneurysm by consensus (over 0.5 cm/6 months) [8-10]. Maximum size of the aorta in large series regarding treatment of aortic aneurysms is 10 cm [3]. We describe a case of a 70 year old man presenting with a rapidly expanding descending aortic aneurysm of 12 cm in diameter at the time of surgical intervention, which had enlarged rapidly within one year, for the first time in the literature.
Case Presentation
A 70 year old man presented with a descending thoracic aortic aneurysm that was diagnosed 1 month before presentation with CT scan for investigation of low back pain. His past medical history was unremarkable, except for the presence of arterial hypertension, which was controlled successfully with antihypertensive medication. The size of the aneurysm at the initial CT scan was 10 cm in diameter and it was evident at the chest X ray as an obvious bulging beside the left heart border, which was misinterpreted as lung atelectasis by the pulmonologist the patient was initially referred (Figure 1). The aneurysm was not evident in a chest X ray that the patient had undertaken one year ago (Figure 2). The patient was subjected to a new CT scan, with intravenous contrast, which showed the size of the aneurysm to have reached a diameter of 12 cm and a prominent tortuosity of the aorta (Figure 3). He was admitted to the hospital for blood pressure control with intravenous glyceryl trinitrate (Nitrolingual) (systolic blood pressure was maintained strictly below 110 mm Hg) and was scheduled for urgent surgery.
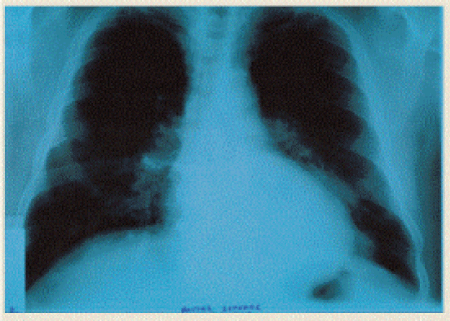
Figure 1: The aneurysm is evident at the chest X ray as an obvious bulging beside the left heart border.
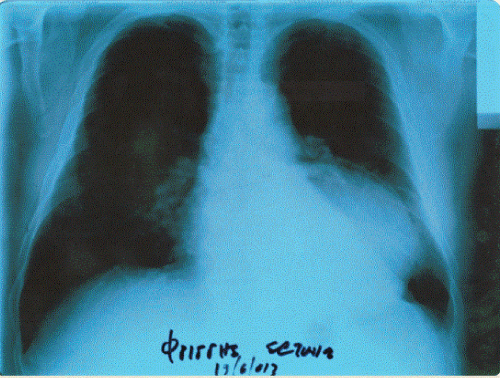
Figure 2: The aneurysm was not evident in a chest X ray, the patient had undertaken one year ago.
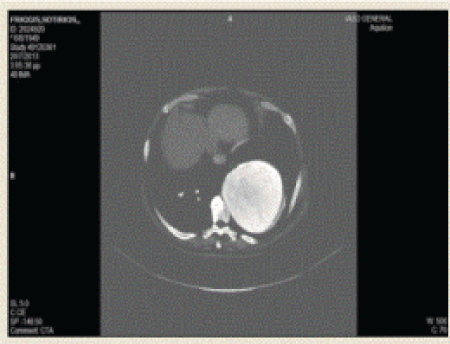
Figure 3: Chest CT scan showing the aneurysm of the descending thoracic aorta.
Surgery was undertaken at the angiography suite. The method of general anesthesia was chosen as the anesthetic modality. Before administering general anesthesia, a lumbar catheter was inserted at the L4-L5 space, in case cerebrospinal fluid (CSF) drainage was necessary postoperatively for reversing potential postoperative spinal cord complications. The rationale for inserting the lumbar drainage catheter prophylactically was that the patient was at a higher risk for developing paraplegia after surgery, because of the necessity of covering the entire descending aorta with the stent graft, as was judged preoperatively due to the large size of the aneurysm, but we did not consider it necessary to start CSF drainage before or during surgery.
The left femoral artery was chosen for the stent graft insertion, because of better morphology and larger size, and it was exposed by a vertical incision at the groin. The left main femoral artery as well as its main branches, that is the superficial and the deep femoral arteries were dissected and controlled.
Under angiographic guidance a stent graft (Medtronic Valiant Captivia 36 mm × 200 mm) was inserted over the guide wire and was deployed on zone 3 (Figure 4), during an episode of purposeful lowering of the systolic blood pressure to 100 mm Hg by the anesthetist. There was no evidence of endoleak after deployment. The left femoral artery was formally repaired after the completion of the endovascular part of the operation by inserting single Prolene 6-0 sutures, supplemented by an additional adventitial running prolene stitch, due to the relatively arteriosclerotic nature of the femoral artery.
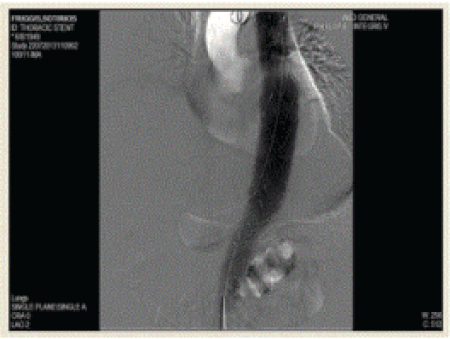
Figure 4: Under angiographic guidance a stent graft was inserted over the guide wire and was deployed on zone 3.
The patient tolerated the procedure perfectly well and was extubated at the angiography suite. He was transferred to the ward. Fluid balance was carefully monitored, maintaining adequate hydration while volume overload was avoided. A Foley catheter remained in place for 24 hours to allow for accurate measurement of urine output. His mean blood pressure was maintained above 80 mm Hg, by judicious use of his antihypertensive medication, in order to augment spinal cord perfusion and careful and frequent monitoring of lower extremity vascular and neurologic status was paramount. He was mobilized on the first postoperative day. His postoperative cource was uneventful, with no ischemic leg or paraplegia or other complications except fever of 38° Celcius and groin hematoma. The lumbar drainage catheter was removed on the second postoperative day. The patient was discharged home at the 3rd postoperative day. He is well with no evidence of endoleak 5 months after surgery (Figure 5).
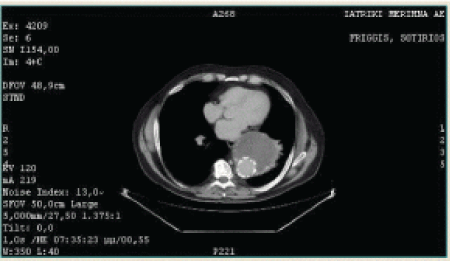
Figure 5: Chest CT scan 5 months after surgery, showing the stent graft inside the descending aorta.
Discussion
Endovascular treatment of descending thoracic aneurysms is commonly performed in patients of older age with comorbid situations, but now it is considered the treatment of choice in most patients, depending on the morphology of the aneurysm. The contraindications for endovascular repair of thoracic aneurysms are related in large to the length and morphology of the landing zones, that is the aorta proximally and distally to the aneurysm, where the stent graft is going to be fixed. Excessive thoracic aorta tortuosity (as there was in the case we present) is not considered an absolute contraindication to endovascular repair [11]. CT scan with intravenous contrast administration is considered the diagnostic and preoperative modality of choice. Endovascular treatment of aneurysms involving the proximal descending aorta sometimes require covering the origin of the left subclavian artery with the stent graft, but that was not the case in the patient we present. The endovascular treatment procedure may be carried out in the operating theatre or the angiography suite and is most commonly performed by exposing either of the femoral arteries at the groin, depending on the morphology and size of these arteries as well as the iliac arteries and the presence or absence of arteriosclerotic plaques at the access vessel. Either local or epidural anesthesia may be used, by we favor general anesthesia, because of the most comfortable and perfect surgical situation it offers.
Published studies suggest that operative repair should be considered when the size of an isolated descending thoracic aortic aneurysm in average risk patients is in the range of 6.5 cm, or 6.0 cm in patients with a family history of Marfan syndrome. Repair is also suggested for patients with documented aneurysm growth of >1 cm per year. While the mean rate of growth of thoracic aneurysms is up to 0.42 cm/year, we are not aware of such a tremendous rate of growth in the literature, as in the case we present.
The risk of morbidity or death is lower with endovascular treatment compared with open repair. The avoidance of aortic cross clamping reduces the risk of end organ damage from ischemia and ischemia–reperfusion. Cardiac, respiratory, renal and peripheral vessels complications are significantly lower than for open surgery [12,13].
Paraplegia is the most feared complication of endovascular repair of thoracic aortic disease. The incidence of spinal cord ischemia (SCI) after TEVAR is generally less when compared to open surgical repair but still occurs with a reported incidence of 0% to 13% [14,15]. Routine use of cerebrospinal fluid (CSF) drainage is not considered mandatory in most cases of TEVAR, but the current trend is towards selective CSF drainage in higher risk patients, or just prophylactic placement of a lumbar catheter without CSF drainage, unless postoperative neurological complications render it mandatory, whereas blood pressure augmentation alone seems to work quite well in case neurological complications arise after TEVAR [16]. Therefore we favored towards inserting a lumbar catheter prophylactically for CSF drainage in case the patient developed signs of CSI after surgery, with the rationale of simplifying the postoperative monitoring and we removed it 48 hours after operation.
Conclusion
Aortic size is considered to be the most important factor regarding the decision towards surgical intervention on a nonemergent versus emergent basis, as was the case in the patient we describe. In one study of descending aortic aneurysms, the aneurysm ruptured at sizes over 10 cm in 8 out of 9 cases [17], which justifies our decision to perform intervention urgently in the case we present, whereas in a large series the median size of the descending aorta at the time of rupture was 8 cm [18]. The risk of rupture must be weighed against anesthesia and other perioperative risks, such that the benefit of repair outweighs the risk of the surgical procedure.
8963
References
- Bickerstaff LK, Pairolero PC, Hollier LH (1982) Thoracic aortic aneurysms: a population based study. Surgery 92:1103-1108.
- Clouse WD, Hallett JWJr, Schaff HV, Gayari MM, Ilstrup DM, et al. (1998) III Improved prognosis of thoracic aortic aneurysms: a population-based study. JAMA 280:1926-1929.
- Coady MA, Rizzo JA, Hammond GL (1999) Surgical intervention criteria for thoracic aortic aneurysms: a study of growth rates and complications. Ann Thorac Surg 67:1922-1926.
- Shores J, Berger KR, Murphy EA (1994) Progression of aortic dilatation and the benefit of long-term β-adrenergic blockage in Marfan’s Syndrome. N Engl J Med 330:1335-1341.
- Hirose Y, Hamada S, Takamiya M, Imakita S, Naito H,et al. (1992) Aortic aneurysms (growth rates measured with CT). Radiology 185:249-252.
- Matsumura JS, Cambria RP, Dake MD, Moore RD, Svensson LG, et al.(2008) TX2 Clinical Trial Investigators. J Vasc Surg 47:247.
- Makaroun MS, Dillavou ED, Wheatley GH, Cambria RP (2008) Gore TAG Investigators. J VascSurg 47:912.
- Svensson LG, Kouchoukos NT, Miller DC (2008) Society of Thoracic Surgeons Endovascular Surgery Task Force.Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann ThoracSurg85:S1-S41.
- Hiratzka LF, Bakris GL, Beckman JA (2010) ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation121:e266-e369.
- Martin Grabenwoger, Fernando Alfonso, Jean Bachet (2012)Thoracic Endovascular Aortic Repair (TEVAR) for the treatment of aortic diseases: a position statement from the European Association for Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). European Heart Journal 33: 1558-1563.
- Findeiss LK, Cody ME (2011) Endovascular Repair of Thoracic Aortic Aneurysms. SeminInterventRadiol 28: 107-117.
- Conrad MF, Ergul EA, Patel VI, Paruchuri V, Kwolek CJ,et al. (2010) Management of diseases of the descending thoracic aorta in the endovascular era: a Medicare population study. Ann Surg 252: 603-610.
- Gopaldas RR, Huh J, Dao TK (2010) Superior nationwide outcomes of endovascular versus open repair for isolated descending thoracic aortic aneurysm in 11,669 patients. J Thorac Cardiovasc Surg 140:1001-1010.
- Xenos ES, Minion DJ, Davenport DL (2009) Endovascular versus open repair for descending thoracic aortic rupture: institutional experience and meta-analysis. Eur J CardiothoracSurg35:282-286.
- Feezor RJ, Lee WA (2009) Strategies for detection and prevention of spinal cord ischemia during TEVAR. Semin Vasc Surg 22:187-192.
- Ullery BW, Cheung AT, Fairman RM (2011) Risk factors, outcomes, and clinical manifestations of spinal cord ischemia following thoracic endovascular aortic repair. J Vasc Surg 54:677-684.
- McNamara JJ, Pressler V (1978) Natural history of arterio-sclerotic thoracic aortic aneurysms. Ann Thoracic Surg26:468-473.
- Crawford ES, Hess KR, Cohen ES (1991) Ruptured aneurysms of the descending thoracic and thoracoabdominal aorta (Analysis according to size and treatment). Ann Surg 213:417-425.










