Keywords
Erectile dysfunction; epilepsy; antiepileptic drugs; depression; anxiety.
Abbreviations
GTC: Generalized tonic-clonic; AEDs, antiepileptic drugs; CBZ: carbamazepine; VPA: valproate; EI-AEDs: enzyme inducer antiepileptic drugs; TLE: temporal lobe epilepsy; SHBG: sex hormone binding globulin; IIEF-5: International Index of Erectile Function questionnaire – 5 items version; BDI-II: Beck Depression Inventory (2nd edition); HAM-A: Hamilton Anxiety Rating Scale.
Introduction
Epilepsy is one of the most common chronic medical illnesses [1]. Men with epilepsy have an approximately five-folds increase in risk of sexual dysfunction compared to general population [2]. In general, sexual disorders are common in people with epilepsy, occurring in up to 1/2-2/3 of patients [3-6]. Various aspects of sexual functions are affected in men with epilepsy including sexual interest and poor sexual performance (as diminished Libido, potency, or satisfaction of erection or orgasm and premature ejaculation) [7]. Erectile dysfunction (ED) is defined as a failure to persistent or recurrent partial or complete obtaining and/or maintaining penile erections until the end of sexual activity. The etiology of ED in patients with epilepsy is multifactorial, involving neurological, endocrine, iatrogenic and psychosocial. ED may be due to disturbance of sex hormones, hypothalamic-pituitary axis and testicular function by epileptic discharges [3, 8,9], or may result as adverse effect of antiepileptic drugs (AEDs) [10-12]. Reduced serum levels of free testosterone and/or albumin bound testosterone and free androgen index (FAIs) and increased levels of estradiol (E2), sex hormone binding globulin (SHBG), follicle stimulating hormone (FSH), luteinizing hormone (LH) and prolactin were reported in men with epilepsy [3-6]. Enzyme inducer AEDs (EI-AEDs) as carbamazepine (CBZ), phenytoin (PHT) and phenobarbitone (PB) elevate SHBG and reduce bioactive testosterone levels and may result in impotence in men [13, 14]. Epilepsy signifies anxiety and depression, low self-esteem and immaturity and this could lead to avoid situations that call for affective sexual involvement [15-18].
Aim of work
In this work, we aimed to determine the frequency rate of ED in a cohort of adult men with epilepsy and its relation to demographic-, clinical-, epilepsy-, psychosocial- hormonaland treatment- related variables.
Materials and methods
This study was conducted on 100 adult males with epilepsy, with age ranges between 20 to 48 years and total time of illness ranged from 3 to 35 years. Patients were on regular treatment with one or combined therapy of the conventional AED(s) for at least 6 months before participation in our study. Patients were free from other neurological or medical diseases and had normal computer tomography (CT) or magnetic resonance imaging (MRI) of the brain. Their seizure types’ were diagnosed according to the International League Against Epilepsy (ILAE) [19]. Patients were recruited from the out-patient epilepsy clinics of the departments of Neurology and Psychiatry of Assiut and Al-Azhar University Hospitals, Assiut, Egypt. Fifty healthy males matched for age, sex, educational level and socioeconomic status were included as controls for comparison. Controls were recruited from the healthy general population matched for age- (range: 20-48 years; mean: 30.36±7.59), sex-, educational level and socioeconomic status.
Excluded were subjects (patients and controls) with: 1) history of neurological disorder other than epilepsy, 2) history of clinically significant genitourinary disease, pelvic trauma, pelvic surgery, or radiation therapy, 3) concomitantly known vascular risks (e.g., heavy smoking, diabetes mellitus (DM), coronary artery disease) , systemic illness (e.g. gout, active gastrointestinal disease, renal failure, serum creatinine concentration >150 mmol/l, chronic hepatic illness) likely to affect erectile function, 4) alcoholism or diagnosed substance abuse and/or previous hospitalization for substance abuse), 5) use of regular medication(s) in addition to AEDs (e.g. drugs for high blood pressure, heart medications, antidepressants, tranquilizers, and sedatives), and 6) current or recent treatment of ED with intracorporeal injection or application of vasoactive drugs.
The study protocol was approved by the ethical committee of the faculties of medicine of Assiut and Al-Azhar, Assiut, Egypt and all patients and control subjects gave their informed consent to participate in this study as follow:
Methods
All patients and healthy control subjects underwent the same research protocol. Patients’ evaluation was done interictally,
1) Medical, neurological, endocrinological, psychiatric, vascular and urological history and examination:
Seizure variables included age at onset, precipitating factors, duration of illness, type, frequency, family history of epilepsy, type of utilized AED(s) (monotherapy or polytherapy), duration of treatment, degree of patients’ control on AED(s) and side effects from medications. The frequencies of seizures were defined as described before [20] into: a) very frequent: occuring several times a day or at intervals shorter than 7 days, b) frequent: occuring at intervals longer than 7 days but shorter than 30 days, c) occasional: occuring at intervals longer than 30 days but shorter than one year, and d) rare: occuring at intervals longer than one year. Regarding the degree of control on AEDs, patients were considered controlled on AEDs treatment, when seizure free for ≥1 year, partially controlled when seizure frequencies were occasional and uncontrolled when seizures were frequent or very frequent.
Physical and urological examinations included examination for the presence of secondary sexual character, genitalia, other tissues of the penis and pelvic region, rectal and prostate examination and examination of perianal and penile sensation. Bulbocavernosal reflex was done to test the integrity of the sacral arc, performed by squeezing the glans (head) of the penis, which immediately cause the anus to contract if nerve function is normal. The latency between squeeze and contraction was assessed by observing the anal sphincter or by filling it with a gloved finger inserted past the anus.
All patients underwent standard electroencephalography (EEG) and neuroimaging [as computed tomography (CT) or magnetic resonance imaging (MRI)].
2) Assessment of erectile function:
Assessment of erectile function was done using the Arabic translated version [21] of International Index of Erectile Function questionnaire 5-items version or IIEF-5 [22]. Each item of IIEF-5 is scored from 0 to 5 on 4 items and 1 to 5 on 1 item. The 4 of the 6 items includes questions regarding maintenance ability, erection confidence, maintenance frequency, and erection firmness, while the single item includes question about intercourse satisfaction and overall satisfaction. A cutoff value of 21 is chosen so that patients with IIEF-5 scores of ≤21 are classified as having ED while scores >21 are not. According to this scale, ED is classified into four categories: severe ED (1–7), moderate ED (8–11), mild-to-moderate ED (12–16), mild (17–21), and no ED (22–25).
3) Psychiatric evaluation:
Standardized psychiatric interview was done by applying the Diagnostic and Statistical Manual of Mental Health Disorders, fourth edition (DSM–IV) criteria for the diagnosis of depression and anxiety [23]. A standardized psychiatric interview was chosen as the primary method for obtaining data. A differentiation between behavioral disorders and behavioral symptoms was made throughout the work. In patients with coexisting symptoms, assessment of the severity of the symptoms was undertaken by an experienced psychologist using the Arabic translated versions of Beck Depression Inventory (2nd edition) (BDI-II) [24, 25] and Hamilton Anxiety Rating Scale (HAM-A) [26,27]. BDI-II is the revised version of the original BDI (1st edition) [28]. In BDI-II, the items reflecting somatic disease and consequences of a medical illness which overlap with depressive symptoms were eliminated (as fatigue, work disability, weight loss, loss of energy, sleep loss, appetite loss, changes in body image, and somatic preoccupation). The BDI–II consists of 21 items to assess the intensity of depression. Each of the 21 items corresponding to a symptom of depression is summed to give a single score for the BDI-II. According to summation of scores from all 21 parameters, the score of 0-13 is considered minimal range, 14-19 is mild, 20-28 is moderate, and 29-63 is severe. The HAM-A consists of 14 items to measure the severity of a patients’ anxiety. Each of the 14 items is scored on a 5-point scale, ranging from 0 = not present to 4 = severe. According to summation of scores from all 14 parameters, the total score of 14-17 is considered as mild in range 18-24 as moderate while 25-30 as severe. Each BDI-II and HAM-A test takes ~15-20 minutes to complete the interview and score the result.
4) Laboratory investigations:
Standard laboratory tests included: complete blood count (CBC), measurement of serum creatinine, liver enzymes and fasting blood glucose level. For analysis of serum free testosterone, prolactin, free thyroxin (FT4), thyroid stimulating hormone (TSH) and sex hormone binding globulin (SHBG), blood samples were drawn at (8.00-10.00 am) after an overnight fast and patients were seizure free for at least 72 hours, as any postictal central hormonal dysfunction is recognized to reverse within hours. Assessment protocols were done according to the recommendations of the manufacturers. The concentrations of serum prolactin, FT4 and TSH were measured by IMMULITE reproductive hormone assays from Diagnostic Product Cooperation (Los Angeles, USA). The concentrations of free testosterone were measured by enzyme immunoassay method (ELISA) (BioSource Europe S.A. Rue de I`Industrie, 8 – B-1400 Nivelles – Belgium) and SHBG concentrations were measured by ELISA kits (IBL international GMBH, Hamburg, Germany). For confirmation, the serum hormone levels were obtained and assessed twice at two different days. The hormonal levels were combined with the cross sectional assessment while clinical evaluation and interviewing with patients and control subjects. Compliance to antiepileptic medications was confirmed by assessment of the serum drug level at least once during the period of the study. The serum levels of AEDs were determined in the Therapeutic Drug Monitoring (TDM) lab, Assiut University Hospital, Assiut, Egypt, using fluorescence polarization immunoassay system of Abbott (EPIA), TDxFLX apparatus (Abbott Lab, Wiesbaden, Germany) as described before [29]. The serum levels of AEDs were measured as part of the investigation in batched assays. The approximated therapeutic serum level of CBZ is 4-10 μg/ ml and that of VPA is 50-100 μg/ml.
Statistical analysis
Calculations were done with the statistical package SPSS, version 12.0. Data were presented as mean ± SD (standard deviation) when normally distributed and mean (quartiles) when not normally distributed. Kolmogorov-Simirnov test was used to test the parameter distributed. Unpaired two-sided Student’s t test was used for comparison of mean of normally distributed parameters. In all other cases Mann-Whitney U test was used for comparison. Correlations must be assessed using Pearson’s for normally distributed data and Spearman’s methods for non-normally distributed data. For all tests, values of p<0.05 were considered statistically significant.
Results
This study included 100 adult male patients with primary epilepsy, with mean age of 30.95±6.95 years, and mean duration of illness of 14.11±7.1 years. Patients were treated with one or more AEDs [CBZ (n=51), VPA (n=23) and combined therapy with CBZ+VPA (n=16), CBZ+PHT (n=8) or CBZ+PHT (n=2)]. Fifty eight percent of patients (58/100) had focal seizures with secondary generalization, 53.45% of them (31/58) had complex partial (frontal) and frontal lobe epilepsy with secondary generalization while 39.66% (23/58) had complex partial (temporal) and temporal lobe epilepsy with secondary generalization. Left focal epileptic activity was detected in 60.34% (35/58), while right focal epileptic activity was detected in 39.66% (23/58). The majority of patients (54%) were uncontrolled on AEDs (had frequent or very frequent seizures) while 21% were seizure free for ≥1 year. Table (1) demonstrated the demographic and clinical features of the studied patients.
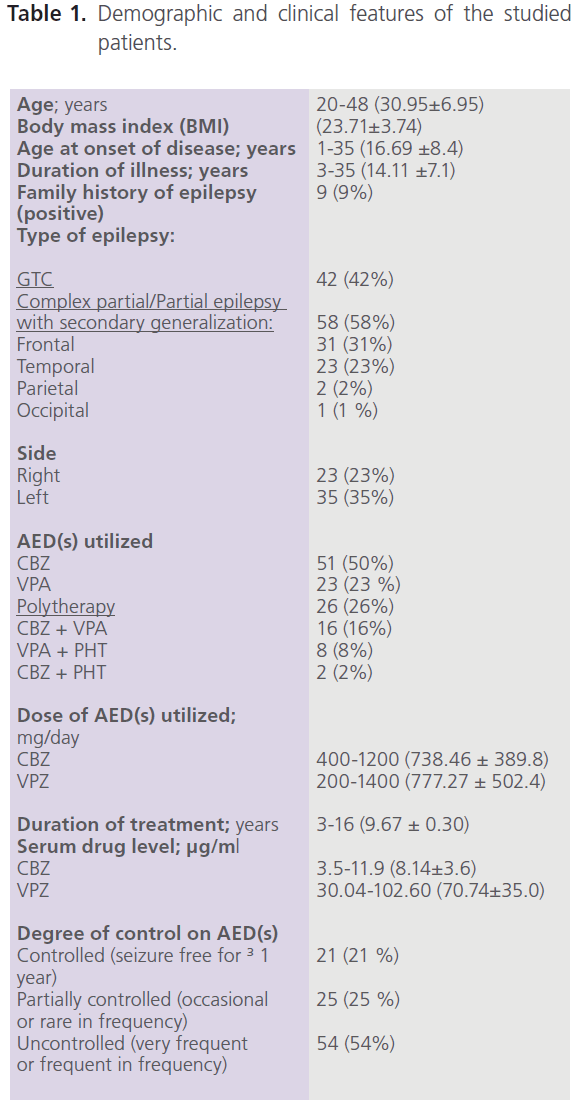
Table 1: Demographic and clinical features of the studied patients.
Erectile function findings
Compared to healthy control subjects, patients with epilepsy had higher frequency rate of ED (37% versus 22%), mostly of mild degree (62.16% or 23/37), 29.73% or 11/37 for moderate; 8.12% or 3/37 for severe degree, and lower scores of IIEF-5 (21.82±4.15 versus 22.98±2.30; P=0.053), particularly patients with focal epilepsy (P=0.042), frontal lobe epilepsy (P=0.040) and left sided foci of epileptic activity (P=0.051). Higher frequencies of ED were observed with CBZ (68.63% or 35/51), followed by polytherapy (34.62% or 9/26) and VPA (26.09% or 6/23). Lower scores of ED were observed with CBZ (20.50±4.96, P=0.005) and those who were uncontrolled on AEDs (21.17±4.55, P=0.012) but not with VPA (22.79±2.86.86, P=0.522) or combined therapy (23.02±3.342.86, P=0.522) (table 2, 3).
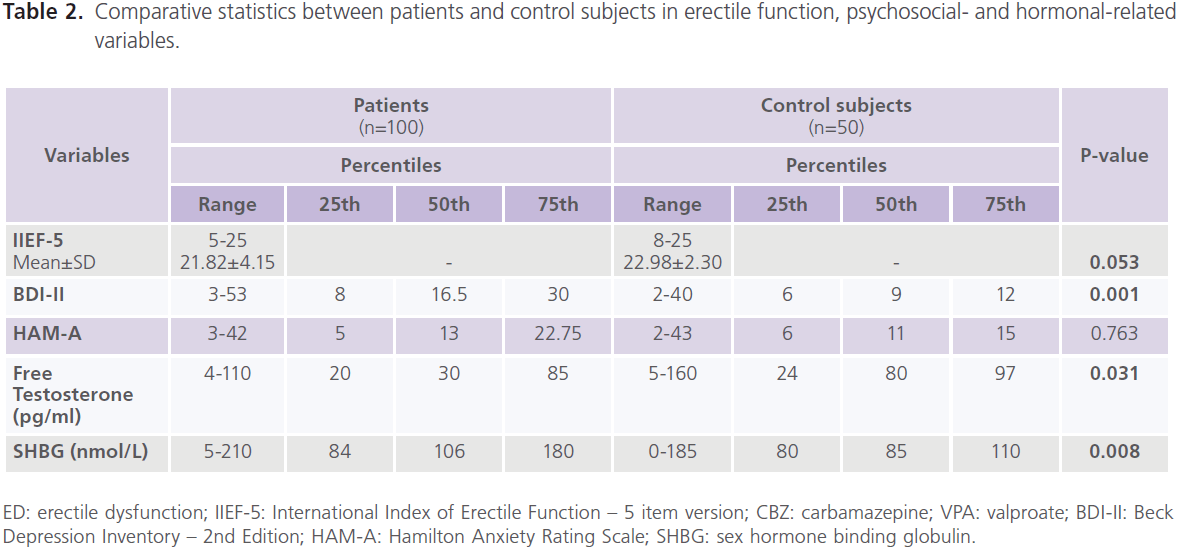
Table 2: Comparative statistics between patients and control subjects in erectile function, psychosocial- and hormonal-related variables.
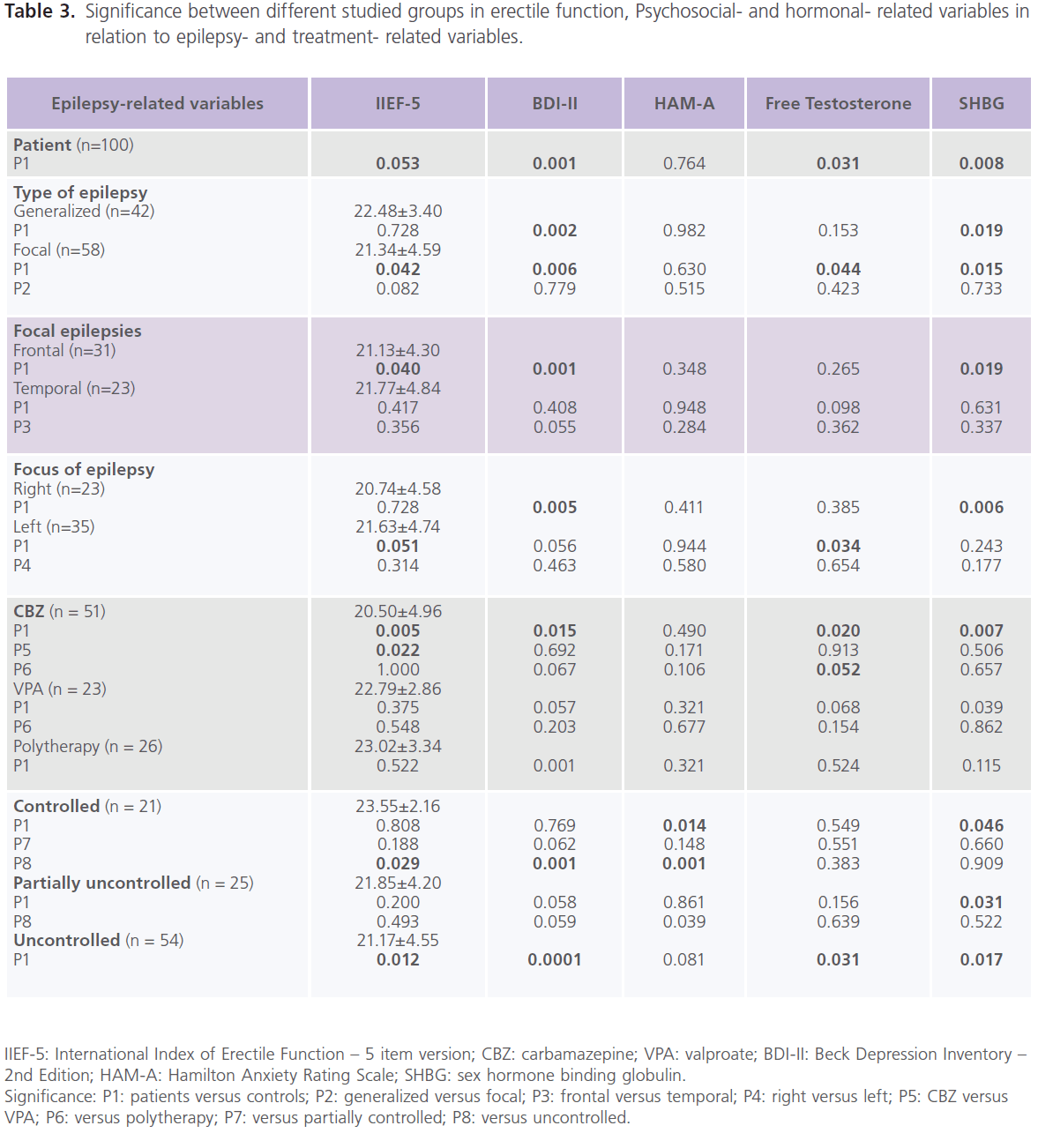
Table 3: Significance between different studied groups in erectile function, Psychosocial- and hormonal- related variables in relation to epilepsy- and treatment- related variables.
Result of testing for depression and anxiety
Compared to healthy control subjects, a high frequency of depression was reported with epilepsy, with 51% meeting criteria for major depressive disorder, mostly of severe degree (72.55%, 37/51) [mild: 15.69%, 8/51; moderate: 11.76%, 6/51)] and higher scores of BDI-II for depression (P=0.001). No significant difference was found between patients with generalized and focal epilepsies in scores of BDI-II, but significantly higher scores were identified with frontal lobe epilepsy (P=0.001) and right sided foci of epileptic activity (P=0.005). Higher scores of BDI-II were observed with CBZ (P=0.015), polytherapy (P=0.001), and those who were uncontrolled on AEDs (P=0.0001) but not with VPA (table 2, 3). Forty percent of patients had manifestations of anxiety, mostly of mild/ moderate type (50% or 20/40). No differences was identified in of scores of HAM-A for anxiety compared to healthy control subjects regardless of seizure type, focus and side or type of AEDs. However, patients who were controlled on AEDs had lower scores of HAM-A (P=0.001) (table 2).
Hormonal findings
Compared to healthy control men, patients with epilepsy had lower serum levels of free testosterone (P=0.034) and higher levels of SHBG (P=0.008). Significantly lower levels of free testosterone were observed with focal epilepsy (P=0.044) and left sided foci of epileptic activity (P=0.008). Significantly higher levels of SHBG were observed with generalized (P=0.019) and focal epilepsies (P=0.015) particularly of
Values are expressed as range; mean ± SD and number (%). GTC: generalized tonic-clonic; AEDs: antiepileptic drugs; CBZ: carbamazepine; VPA: valproate; PHT: phenytoin.
frontal lobe type (P=0.019) and those with right sided foci of epileptic activities (P=0.006). Significantly lower levels of free testosterone were observed with CBZ (P=0.020) and those who were uncontrolled on AEDs (P=0.031). Significantly higher levels of SHBG were observed with CBZ (P=0.007) and VPA (P=0.039) and patients who were partially controlled (P=0.031) and uncontrolled (P=0.017) on AEDs (table 2, 3). Compared to healthy controls subjects, no significant differences were reported in the serum levels of prolactin
(8.7±3.4 ng/ml versus 9.2±3.6; P=0.118), FT4 (0.39±0.10 ng/ dl versus 0.46±0.29; P=0.651) or TSH (2.23±1.77μIU/ml versus 2.55±0.96; P=0.463).
Table 4 showed that compared to patients without ED, patients with ED were older in age (34.32±7.30 versus 29.14±6.19, P1=0.001), older at age of onset (19.49±9.66 versus 15.05±7.16, P1=0.016), had higher rates of uncontrolled seizures (58.19%) and higher scores of BDI-II (P=0.0001) and HAM-A (P=0.0001).
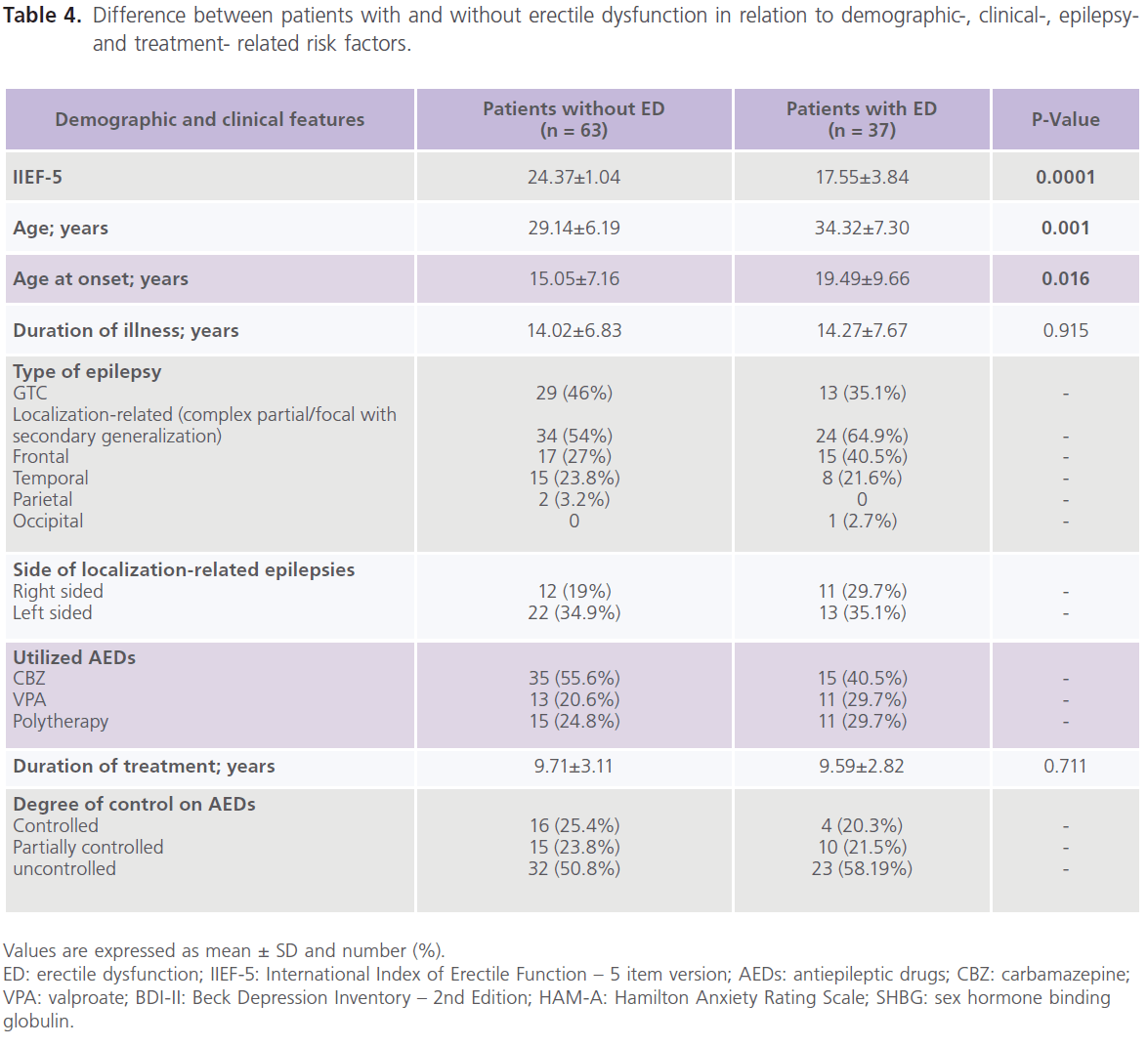
Table 4: Difference between patients with and without erectile dysfunction in relation to demographic-, clinical-, epilepsyand treatment- related risk factors.
Correlations between scores of IIEF-5, demographic-, clinical-, psychosocial-, hormonal-, epilepsy- and treatment- related variables showed that there was a negative significant correlations between ED and age (-0.482, P=0.0001), age at onset (-0.200, P=0.042) and scores of BDI-II (-0.391, P=0.0001) and scores of HAM-A (-0.539, P=0.0001), but not with treatmentand hormonal- related variables. Also scores of BDII were positivity correlated with scores of HAM-A (table 5 and 6).
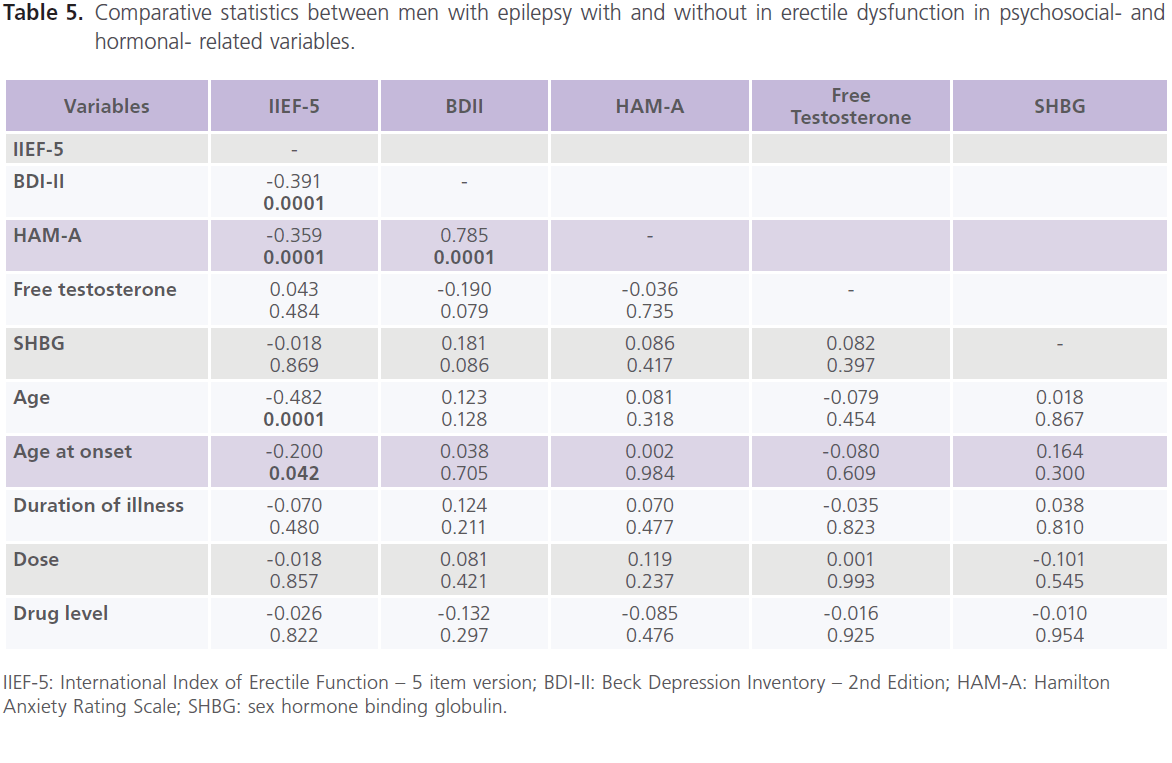
Table 5: Comparative statistics between men with epilepsy with and without in erectile dysfunction in psychosocial- and hormonal- related variables.
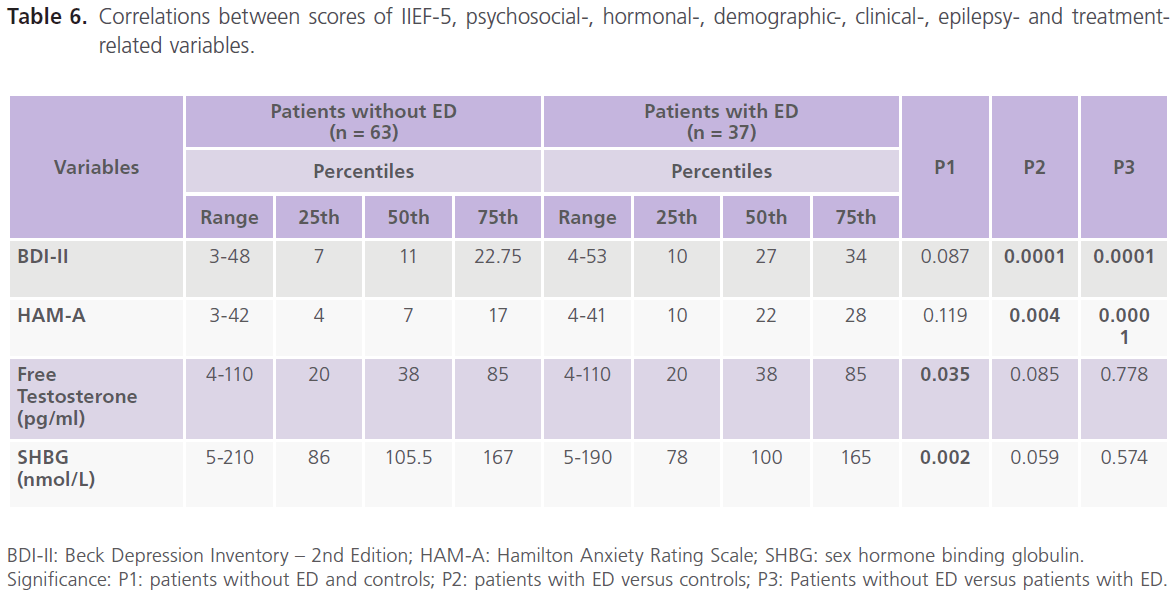
Table 6: Correlations between scores of IIEF-5, psychosocial-, hormonal-, demographic-, clinical-, epilepsy- and treatmentrelated variables.
Discussion
The results of this study indicate that patients with chronic epilepsy are at increased risk of ED (37% versus 22% for healthy control subjects). In accordance, hyposexuality, manifested as diminished sexual interest or decreased libido and poor sexual performance or potency, is the most frequent interictal abnormality previously reported in men with epilepsy (28-70.4%) [2,5,30]. Patients with focal epilepsy had lower scores of ED, particularly those with frontal lobe epilepsy and those with left sided focal epileptic activity. This is in contrast to studies which indicated that sexual dysfunction occurs in patients with partial epilepsies, particularly TLE and amygdala lesion as the limbic system is extensively interconnected with the hypothalamic nuclei involved in regulating gonadal and sexual function and androgen serum levels [31-34]. While others reported that more than 50% of patients with generalized tonic-clonic convulsions may develop reproductive and sexual abnormalities [5,35]. Several studies reported higher frequency of ED in men with generalized [5,35] and partial epilepsy, particularly when followed by secondary generalization [36]. Some studies reported that hyposexuality are more prominent with right than with left temporo-limbic foci [3,37].
Fifty one percent of the studied patients had criteria of major depressive disorder, mostly of severe type (73%) and higher scores of BDI-II (P=0.001) but not in HAM-A (P = 0.944). Depression is the most frequent psychiatric disorder in patients with epilepsy, with prevalence of 20-55% and sometimes up to 80% compared to 1.5-19% in the general population [39-40]. The poor adjustment to seizures, low socioeconomic status, financial stress, poor cultural approach to epilepsy, poorer academic achievement, unemployment (with rates up to 50% in developed countries if seizures are not fully controlled and up to 100% in developing countries) and inability to drive, all result in a negative effect on effort and attitude about his abilities, low self-esteem, lessened well-being, social isolation, stigmatization, marital stresses, psychiatric abnormalities and a lower quality of life [7, 38]. Patients with ED had higher scores of BDI-II than patients without ED (P = 0.0001) [15]. A highly significant correlation was identified between the scores of IIEF-5 and BDI-II
IIEF-5: International Index of Erectile Function – 5 item version; CBZ: carbamazepine; VPA: valproate; BDI-II: Beck Depression Inventory – 2nd Edition; HAM-A: Hamilton Anxiety Rating Scale; SHBG: sex hormone binding globulin. Significance: P1: patients versus controls; P2: generalized versus focal; P3: frontal versus temporal; P4: right versus left; P5: CBZ versus VPA; P6: versus polytherapy; P7: versus partially controlled; P8: versus uncontrolled.
(P = 0.0001) and HAM-A (P=0.0001) and between BDI-II and HAM-A (P=0.0001). The immaturity as a result of the seizure, diminished sexual desire and responsiveness, all can lead to avoid situations that call for affective sexual involvement [15,39,40]. In this study, significant negative correlation was identified between age and age at onset and ED. In fact, it has been found that the earlier onset (below the age of 18) and longer duration of epilepsy, the better quality of life in adult patients. This reflects the more effective copying mechanisms and the adjustment to the social and psychological consequences of the disease. While the late onset seizures may be more disruptive to patients’ life, for example: due to loss of the ability to drive and unemployment [41]. In this study, patients with frontal lobe epilepsy and those with right sided foci of epileptic activity had lower scores of BDI-II. This is in accordance to studies which implicate frontal lobe as a cause of depression even in patients with temporal lobe epilepsy due to association with frontal lobe hypometabolism. In support [42]: 1) a bilateral reduction in inferior frontal lobe glucose metabolism on positron emission tomography (PET) scans was observed in patients with depression and TLE, and 2) reduced activity measured with single photon emission computed tomography (SPECT) in bilateral frontal and right temporal regions was associated with higher scores on the Beck Depression Index in patients with left TLE [43]. Abnormalities of metabolism in the amygdala and anterior cingulate gyrus have also been reported to be associated with depression and TLE [42]. The majority of the studies implicate left sided foci (2-3 folds higher) than right sided foci as a potential risk factor for depression in epilepsy, particularly left sided TLE in association with frontal lobe hypofunction while other studies implicate the right hemisphere as a risk of depression in epileptic patients and suggested that this might be attributed to the extensive limbic connections than the left hemisphere. Others found no effect of lateralization at all [44].
In this study, men with epilepsy had lower serum levels of free testosterone (P=0.031) and higher SHBG (P=0.008) [3,6,13]. No significant difference was identified between patients with and without dysfunction in the serum levels of free testosterone or SHBG. Androgens play an important role in sexual interest and maintenance of libido and sexual potency in men with epilepsy. Decreased level of free testosterone is associated with decreased sexual interest and potency [13]. Free testosterone (bioactive testosterone) represents 2% of total testosterone and the majority of plasma testosterone is linked to albumin (43-45%) and, 53-55% to SHBG [3]. Increased SHBG would expect to produce sexual dysfunction by decreasing serum levels of free testosterone and/or albumin bound testosterone. Also, SHBG is the most important regulator for the biologic effect of the testosterone on the target tissue (i.e. it decreases the activity of the testosterone on the target cells) [2]. Secondary hormonal changes can occur with epilepsy due to negative feedback loop between testosterone and pituitary hormone. Disturbances may occur at the levels of the hypothalamic, pituitary and/or gonadal levels. Significant lowering in serum levels of free testosterone was identified in left sided focal epileptic activity despite the type of epilepsy which is in contrast to several studies which reported lower levels of free testosterone with TLE [2] and normalization of its level in seizure-free patients after temporal lobectomy [33].
In this study, patients on CBZ, had higher frequencies of ED (68.63%), and lower scores of IIEF-5 (P=0.005), higher scores for depression (monotherapy: P=0.015; polytherapy: P=0.001). EI-AEDs are known to reduce folic acid levels which might be implicated as a risk for depression in patients with epilepsy [38]. Folic acid plays a crucial role in several important central nervous system transmethylation reactions and is linked to monoamine metabolism. CBZ is an EI-AED that is principally metabolized in the hepatic P450 system (CYP450), which has a multitude of functions, including the synthesis and breakdown of endogenous substances resulting in metabolic side effects [45]. In this study, patients on CBZ had significantly lower levels of free testosterone (P=0.02) and higher scores of SHBG (P=0.007). Several studies reported that men on CBZ have lower serum testosterone levels, low free androgen index (FAIs), esterone, dehydroepiandrosterone sulfate (DHEAS), androstenedione and testosteroneto- SHBG ratio and increased concentrations of SHBG and these contribute to sexual dysfunction in men with epilepsy [12, 46]. CBZ is a commonly used AED in middle and lower income developing countries. AEDs, EI-AEDs were found to modulate hormone release from the hypothalamic-pituitarygonadal axis and may have direct inhibitory effect on sexual and reproductive functions. EI-AEDs can act through competition of binding plasma protein making the free fraction ready for metabolism. Also EI-AEDs may enhance the conversion of testosterone to estradiol by aromatase [47]. The rise in SHBG and increased androgen catabolism with decrease free testosterone fraction and dihydrotestosterone levels are secondary to induction of hepatic monooxygenase activity by EI-AEDs and this may also result in lower seizure threshold [3,12]. In support, oxcarbazepine (OXZ), a minimally enzyme-inducing analog of CBZ, was not found to be associated with changes in testosterone level [34]. Studies found that CBZ-associated changes in serum sex hormone balance could be avoided by replacing CBZ with OXZ [48]. Others found that replacement of CBZ with OXZ resulted in cure of impotence induced by CBZ [47]. OXZ is metabolized by cytosolic non-microsomal, non-inducible keto-reductase and glycuronosyltransferase. The metabolism is then independent of the cytochrome P450 system regardless whether CYP3A4 and UGT enzyme systems (UGT: UDP-glucuronosyltransfer ase; UDP: uridine 5`-diphosphate; Glucuronidation is a major pathway for xenobiotic biotranformation in mamalian species as drug-drug interaction) are induced by OXZ [50]. Also some authors found that utilization of lamotrigine (LTG) in men with epilepsy, a new AED with no enzyme inducing properties, is associated with normal sexual function, bioavailable testosterone levels, hormone ratios (bioactive testosterone/ bioactive estradiol), and gonadal efficiency (bioactive testosterone/ luteinizing hormone) when compared to men on CBZ or phenytoin (PHT) [45].
The results of this study indicate that the etiology of ED in men with epilepsy is multifactorial. Changes in central control and peripheral hormone levels caused by epilepsy itself [8,15] and its medications [2,10,11,13,14] together with psychosocial consequences, are contributing factors ED in men with epilepsy [3,5,6]. However, the results of this study support the notion that the risk of epilepsy itself over-weighted that of AEDs. In support: 1) Compared to those with good drug responders, poor drug responders were found to have lower scores of ED (P=0.012), lower levels of testosterone (P=0.028), higher levels of SHBG (P=0.017) and higher scores of depression (P=0.031). 2) No relationship had been identified between the dose and drug level of AEDs and scores of ED, scores of depression or anxiety in spite of the fact that CBZ and VPA have been found to increase the synaptic secretion of serotonin and have antidepressant effect [51], 3) The different pattern of sexual dysfunction encountered in different seizure types (i.e. generalized versus localizationrelated) and the lateralization asymmetry of the pattern of sexual dysfunction encountered in some patients further supports that epilepsy itself is the cause of these abnormalities [2], and 4) Satisfaction and sexuality were better in operated epileptics free of seizures [33].
In fact, the relationship between epilepsy and depression, ED and depression are bidirectional, i.e. one disorder may lead to the others. Dysfunctions of the limbic system, frontal– limbic–subcortical circuits, frontal-striatal systems, limbicbrainstem connections or amygdale and its connections (e.g. amygdale-hypothalamic ED amygdale-locus ceruleus) are the most important causes of epilepsy and its related comorbidities as depressive and behavioral symptomatologies and ED [52]. Intractable epilepsy, generalized, temporal and frontal lobe epilepsies (the most frequent types of partial epilepsies) were found to result in hypo-metabolism of temporal as well as extratemporal regions as the frontal lobe. The frontal lobe dysfunction, inter-ictal inhibitory activity, dysfunction of dopaminergic, non-adrenergic, and serotonergic systems, post-ictal depletion of substrates (decreased levels of neurotransmitters) of the limbic-frontal regions or functional differentiation, increase seizure intractability to medications and vulnerability to depression and ED [5,16,17,51].
Conclusions
This study indicates that psychiatric comorbidity (as depression and anxiety) and reproductive endocrine abnormalities appear to be related to ED. It is plausible that ED and reproductive endocrine abnormalities may furthermore promote the seizure intractability to AED. Attention should be paid to optimize seizure control as well as changing dysfunctional thoughts and behaviors and interactions that are found to inhibit sexual arousal. Regular psychiatric consultation and psychotherapy are sometimes needed. In addition, modalities such as medications for ED may be useful including aromatase inhibitors and sildenafil.
Authors’ disclosure
None
6465
References
- Hauser, WA., Annegers, JF., Kurland, LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935-1984. Epilepsia 1993; 34 (3): 453-468.
- Herzog, AG., Seibel, MM., Schomer, DL., Vaitukaitis, JL., Geschwind, N. Reproduction endocrine disorders in men with partial seizures of temporal lobe origin. Arch Neurol 1986; 43 (4): 347-350.
- Murialdo, G., Galimberti, CA., Fonzi, S., Manni, R., Costelli, P., Parodi, C., Solinas, GP., Amoretti, G., Tartara, A. Sex hormones and pituitary function in male epileptic patients with altered or normal sexuality. Epilepsia 1995; 36 (4): 360-365.
- Herzog, AG., Coleman, AE., Jacobs, AR., Klein, P., Friedman, MN., Drislane, FW., Schomer, DL. Relationship of sexual dysfunction to epilepsy laterality and reproductive hormone levels in women. Epilepsy Behav 2003; 4 (4): 407-413.
- Hamed, SA., Mohamed, KA., Taher El., Enas, AM., Hamed, EA., Omar, H. The sexual and reproductive health in men with generalized epilepsy: a multidisciplinary evaluation. IJIR 2006; 18 (3): 287-295.
- Kuba, R., Pohanka, M., Zákopcan, J., Novotná, I., Rektor, I. Sexual dysfunctions and blood hormonal profile in men with focal epilepsy. Epilepsia 2006; 47 (12): 2135-2140.
- Harden, CL. Sexuality in men and women with epilepsy. CNS Spectr 2006; 11 (3): 13-18.
- Leiderman, DB., Csernansky, JG., Moses, JA. Jr. Neuroendocrinology and limbic epilepsy: relationships to psychopathology, seizure variables, and neuropsychological function. Epilepsia 1990; 31 (3): 270-274.
- Montouris, G., Morris, GL. Reproductive and sexual dysfunction in men with epilepsy. Epilepsy Behav 2005; 7 (suppl. 2): S7-14.
- Dana-Haeri, J., Oxley, J., Richins, A. Reduction of free testosterone by antiepileptic drugs. Br Med J (Clin Res Ed). 1982; 284 (6309): 85-86.
- Duncan, S., Blacklaw, J., Beastall, GH., Brodie, MJ. Antiepileptic drug therapy and sexual function in men with epilepsy. Epilepsia 1999; 40 (2): 197-204.
- Røste, LS., Taubøll, E., Mørkrid, L., Bjørnenak, T., Saetre, ER., Mørland, T., Gjerstad, L. Antiepileptic drugs alter reproductive endocrine hormones in men with epilepsy. European Journal of Neurology 2005; 12 (2): 118-124.
- Toone, BK., Wheeler, M., Nanjee, M., Fenwick, P., Grant, R. Sex hormones, sexual activity and plasma anticonvulsant levels in male epileptics. J Neurol Neurosurg Psychiatry 1983; 46 (9): 824-826.
- Isojarvi, JI., Repro, M., Pakarinen, AJ., Ylipalosaari, PJ., Myllyla, VV. Carbamazepine, phenytoin, sex hormones and sexual function in men with epilepsy. Epilepsia 1994; 36 (4): 366-370.
- Smith, DF., Baker, GA., Dewey, M., Jacoby, A., Chadwick, DW. Seizure frequency, patient-perceived seizure severity and the psychosocial consequences of intractable epilepsy. Epilepsy Res 1991; 9 (3): 231- 241.
- Torta, R., Keller, R. Behavioral, psychotic, and anxiety disorders in epilepsy: etiology, clinical features, and therapeutic implications. Epilepsia 1999; 40 (suppl. 10): S2-S20.
- Kanner, AM. Depression and epilepsy: a new perspective on two closely related disorders. Epilepsy Currents 2006; 6 (5): 141-146.
- Beyenburg, S., Mitchell, AJ., Schmidt, D., Elger, CE., Reuber, M. Anxiety in patients with epilepsy: systematic review and suggestions for clinical management. Epilepsy Behav 2005; 7 (2): 161-171.
- Bancaud, J., Henriksen, O., Rubio, F., Masakatsu, S., Fritz, E., Kiffin, P. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia 1981; 22 (4): 489-501.
- Hamed, SA., Hamed, EA., Kandil, MR., El-Shereef, HK., Abdellah, MM., Omar, H. Serum thyroid hormone balance and lipid profile in patients with epilepsy. Epilepsy Research 2005; 66 (1-3): 173-183.
- Shamloul, R., Ghanem, H., Abou-zeid, A. Validity of the Arabic version of the sexual health inventory for men among Egyptians. IJIR 2004; 16 (5): 452-455.
- Rosen, RC., Cappelleri, JC., Smith, MD., Lipsky, J., Peña, BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. IJIR 1999; 11 (16): 319-326.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association. 1994. pp. 317-391.
- Gharyb, AG. Beck Depression Inventory II (BDI-II): Arabic examiner’s handbook. Cairo: Dar El-Anglo. 2000.
- Beck, AT., Steer, RA., Ball, R., Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of personality assessment 1996; 67 (3): 588-597.
- Lotfy, F. Hamilton Anxiety Rating Scale (HAM-A) Cairo: Dar El-Anglo. 1994.
- Hamilton, M. The assessment of anxiety states by rating. Br J Med Psychol 1959; 32: 50-55.
- Beck, AT., Rial, WY., Rickets, K. Short form of depression inventory: cross-validation. Psychol Rep 1974; 34 (3): 1184-1186.
- Goma, A., Abdel-Regal, S., Abdellah, M., Hamed, S. Evaluation of therapeutic drug monitoring of Valproic acid: A 6 years’ experience in Upper Egypt. J Egypt Soc Pharmacol Exp 2004; 25: 431-452.
- Fenwick, PB., Toone, BK., Wheeler, MJ., Nanjee, MN., Grant, R., Brown, D. Sexual behavior in a centre for epilepsy. Acta Neurol Scand 1985; 71 (6): 428-435.
- Quigg, M., Kiely, JM., Shneker, B., Veldhuis, JD., Bertram, EH. 3rd. interictal and postictal alterations of pulsatile secretions of luteinizing hormone in temporal lobe epilepsy in men. Ann Neurol 2002; 51 (5): 559-566.
- Bauer, J., Blumenthal, S., Reuber, M., Stoffel-Wagner, B. Epilepsy syndrome, focus, location, and treatment choice affect testicular function in men with epilepsy. Neurology 2004; 62 (2): 1243-246.
- Baird, AD., Wilson, SJ., Bladin, PF., Saling, MM., Reutens, DC. The amygdala and sexual drive: insights from temporal lobe epilepsy surgery. Ann Neurol 2004; 55 (1): 87-96.
- Pack, A. Effects of treatment on endocrine function in patients with epilepsy. Curr Treat Options Neurol 2005; 7 (4): 273-280.
- Bilo, L., Meo, R., Nappi, C., Annunziato, L., Striano, S., Colao, AM., Merola, B., Buscaino, GA. Reproductive endocrine disorders in women with primary generalized epilepsy. Epilepsia 1988; 29 (5): 612-619.
- Hamed, SA. Neuroendocrine hormonal conditions in epilepsy: relationship to reproductive and sexual functions. Neurologist 2008; 14 (3): 157-169.
- Daniele, A., Azzoni, A., Bizzi, A., Rossi, A., Gainotti, G., Mazza, S. Sexual behavior and hemispheric laterality of the focus in patients with temporal lobe epilepsy. Biol Psychiatry 1997; 42 (7): 617-624.
- Lambert, MV., Robertson, MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia 1999; 40 (suppl. 10): S21- 47.
- Hamed, SA., Metwaly, NA., Hassan, MM., Mohamad, KA., Ahmad, MA., Soliman, AA., Elsaid, AM. Depression in adults with epilepsy: Relationship to psychobiological variables. WJN 2012;2(1):1-10.
- Hamed SA, Elserogy YE, Abduo MA, Abdellah MM. The risks of suicidality in adult patients with epilepsy. WJP 2012; 2(2): 33042. doi: 10.5498/WJP.v2.i2.33.
- Perrine, K., Hermann, BP., Meador, KJ., Vickrey, BG., Cramer, JA., Hays, RD. The relationship of neuropsychological functioning to quality of life in epilepsy. Arch Neurol 1995; 52 (10): 997-1003.
- Richardson, EJ., Griffith, HR., Martin, RC., Paige, AL., Stewart, CC., Jones, J., Hermann, BP., Seidenberg, M. Structural and functional neuroimaging correlates of depression in temporal lobe epilepsy. Epilepsy Behav 2007; 10 (2): 242-249.
- Shih, JJ., Weisend, MP., Sanders, JA., Lee, RR. Magnetoencephalographic and magnetic resonance spectroscopy evidence of regional functional abnormality in mesial temporal lobe epilepsy. Brain Topogr 2011; 23 (4): 368-374.
- Gilliam, FG., Maton, BM., Martin, RC., Sawrie, SM., Faught, RE., Hugg, JW., Viikinsalo, M., Kuzniecky, RI. Hippocampal 1H-MRSI correlates with severity of depression symptoms in temporal lobe epilepsy. Neurology 2007; 68 (5): 364-368.
- Herzog, AG., Drislane, FW., Schomer, DL., Pennell, PB., Bromfield, EB., Dworetzky, BA., Farina, EL., Frye, CA. Differential effects of antiepileptic drugs on sexual function and hormones in men with epilepsy. Neurology 2005; 65 (7): 1016-1020.
- Rättyä, J., Turkka, J., Pakarinen, AJ., Knip, M., Kotila, MA., Lukkarinen, O., Myllylä, VV., Isojärvi, JI. Reproductive effects of valproate, carbamazepine, and oxcarbazepine in men with epilepsy. Neurology 2001; 56 (1): 31-36.
- Petra, P. The plasma sex steroids binding protein (SBP or SHBG). A critical review of recent developments on the structure, molecular biology and function. J Steroid Biochem Mol Biol 1991; 40 (4-6): 735- 773.
- Isojärvi, JI., Pakarinen, AJ., Rautio, A., Pelkonen, O., Myllylä, VV. Serum sex hormone levels after replacing carbamazepine with oxcarbazepine. Eur J Clin Pharmacol 1995; 47 (4): 461-464.
- Bóné, B., Janszky, J. Epilepsy and male sexual dysfunction: etiology, diagnosis and therapy. Ideggyogy Sz 2006; 59 (5-6): 148-152.
- Schachter, SC. Oxcarbazepine: current status and clinical applications. Expert Opinion on Investigational Drugs 1999; 8 (7): 1103-1112.
- Jobe, PC., Dailey, JW., Wernicke, JF. A noradrenergic and serotoninergic hypothesis of the linkage between epilepsy and affective disorders. Crit Rev Neurobiol 1999; 13 (4): 317-356.
- Drevets, WC. Functional neuroimaging studies of depression: the anatomy of melancholia. Annu Rev Med 1998; 49: 341-361.











