Keyword
|
| |
| Ehthosomes, Transdermal delivery, Carriers |
| |
INTRODUCTION
|
| |
| Transdermal drug delivery offers many advantages as compared to traditional drug delivery systems, including oral and parenteral drug delivery system. Advantages claimed are increased patient acceptability (non invasiveness), avoidance of gastrointestinal disturbances which the drug permeates through various layers of skin, via a passive diffusion pathway. However, this limits the basic potential of these systems, as stratum corneum is the most formidable barrier to the passage of most of the drugs, except for highly lipophilic, low molecular weight drugs. To overcome the stratum corneum barrier, various mechanisms have been investigated, including use of chemical or physical enhancers, such as iontophoresis, sonophoresis, etc. Liposomes, niosomes, transferosomes and ethosomes also have the potential of overcoming the skin barrier and have been reported to enhance permeability of drug through the stratum corneum barrier. |
| |
| The vesicles have been well known for their importance in cellular communication and particle transportation for many years. Researchers have understood the properties of vesicles structure for use in better drug delivery within their cavities, which would to tag the vesicle for cell specificity. One of the major advances in vesicle research was the finding a vesicle derivatives, known as an Ethosomes. [2] |
| |
| Ethosomes are noninvasive delivery carriers that enable drugs to reach the deep skin layers and/or the systemic circulation. These are soft, malleable vesicles tailored for enhanced delivery of active agents. They are composed mainly of phospholipids, (phosphatidylcholine, phosphatidylserine, phosphatitidic acid), high concentration of ethanol and water. [3] The high concentration of ethanol makes the ethosomes unique, as ethanol is known for its disturbance of skin lipid bilayer organization; therefore, when integrated into a vesicle membrane, it gives that vesicle the ability to penetrate the stratum corneum. Also, because of their high ethanol concentration, the lipid membrane is packed less tightly than conventional vesicles but has equivalent stability, allowing a more malleable structure and improves drug distribution ability in stratum corneum lipids. |
| |
| The Ethosomes were found to be suitable for various applications within the pharmaceutical, biotechnology, veterinary, cosmetic, and nutraceutical markets. These ?soft vesicles? represents novel vesicular carrier for enhanced delivery to/through skin. The size of Ethosomes vesicles can be modulated from tens of nanometers to microns. |
| |
THE ETHOSOME TECHNOLOGY
|
| |
| Novel Therapeutic technology Inc. (NTT) has exclusively in-licensed and further developed a patented, passive, noninvasive, transdermal drug delivery technology known as the ETHOSOME? Delivery System. Ethosomes were designed to enhance the delivery of drugs into the deep layers of the skin and through the skin. Depending on the formulation, delivery can be targeted for local delivery or for systemic use. The structure of an Ethosome allows it to carry a wide variety of molecules with various physico-chemical properties. A number of these addressing different indications have been formulated and tested in laboratory studies. |
| |
| Ethosomes systems were found to be significantly superior at delivering drugs through the skin in terms of both quantity and depth when compared to liposomes and to many commercial transdermal and dermal delivery systems. Ethosomes are sophisticated vesicular delivery carriers that are capable of delivering various chemical applications. Visualization by dynamic light scattering showed that Ethosomes could be unilamellar or multilamellar through to the core. The size of Ethosomes can be modulated to range anywhere from 30 nm to a few microns. These novel delivery systems contain soft phospholipid vesicles in the presence of high concentrations of ethanol. |
| |
MECHANISM OF DRUG PENETRATION
|
| |
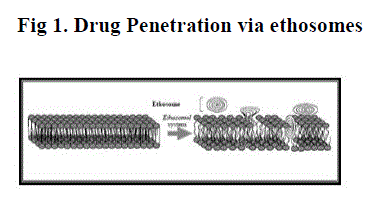
| The enhanced delivery of actives using ethosomes over liposomes can be ascribed to an interaction between ethosomes and skin lipids. A possible mechanism for this interaction has been proposed. It is thought that the first part of the mechanism is due to the ?ethanol effect?, whereby intercalation of the ethanol into intercellular lipids increasing lipid fluidity and decreases the density of the lipid multilayer. [5] This is followed by the ?ethosome effect?, which includes inter lipid penetration and permeation by the opening of new pathways due to the malleability and fusion of ethosomes with skin lipids,resulting in the release of the drug in deep layers of the skin. |
| |
PREPARATION AND CHARACTERIZATION
|
| |
| Ethosomes can be prepared from soybean phosphatidylcholine (Phospholipon 90), ethanol, drug and distilled water. Phospholipon 90 and drug should be dissolved in ethanol. Water has to be added in small quantities and the preparation mixed by mechanical stirring under controlled conditions. [6] |
| |
ADVANTAGES OF ETHOSOMAL DRUG DELIVERY
|
| |
| 1. Ethosomes are enhanced permeation of drug through skin for transdermal and dermal delivery. |
| |
| 2. Ethosomes are platform for the delivery of large and diverse group of drugs (peptides, protein molecules) |
| |
| 3. Ethosome composition is safe and the components are approved for pharmaceutical and cosmetic use. |
| |
| 4. Low risk profile- The technology has no large-scale drug development risk since the toxicological profiles of the ethosomal components are well documented in the scientific literature. |
| |
| 5. High patient compliance- The Ethosomal drug is administrated in semisolid form (gel or cream), producing high patient compliance by is high. In contrast, Iontophoresis and Phonophoresis are relatively complicated to use which will affect patient compliance. |
| |
| 6. High market attractiveness for products with proprietary technology. Relatively simple to manufacture with no complicated technical investments required for production of Ethosomes. |
| |
| 7. The Ethosomal system is passive, non-invasive and is available for immediate commercialization. |
| |
| 8. Various application in Pharmaceutical, Veterinary, Cosmetic field. |
| |
THERAPEUTIC APPLICATION OF ETHOSOMES
|
| |
| Horwitz et al. reported that a 5 % acyclovir ethosomal preparation compared to the 5 % acyclovir cream showed significant improvements in treatment of herpetic infections. |
| |
| Jain et al. prepared zidovudine ethosomes and characterized in vitro and in vivo. The effect of different formulation variables on skin permeation of zidovudine was studied using locally fabricated Keshry-Chien type of diffusion cell. To understand the mechanism of better skin permeation of ethosomes, vesicle skin interaction study was carried out. To confirm the better skin permeability of ethosomes, fluorescence microscopy using rhodamine 123 as fluorescence probe was performed. The optimized ethosomes showed transdermal flux of 78.5±2.5 µg/cm 2 /h across the rat skin. Vesicle skin interaction study showed that ethosomes affected the ultrastructure of the stratum corneum as distinct regions with lamellar stacks derived from 5the vesicles were observed in the intercellular spaces of the stratum corneum. Thus ethosomes can increase the transdermal flux, prolong the release and present an attractive route for sustained delivery of Zidovudine. |
| |
| Dayan et al. investigated the delivery of trihexyphenidyl HCl (THP) from ethosomes versus classic liposomes. As the THP concentration was increased from 0 to 3%, the size of the vesicles decreased from 154 to 90 nm. This is most likely due to the surface activity of THP (critical micelle concentration of 5.9 mg/ml), as measured in this work. In addition, the ethosome zeta potential value increased as a function of THP concentration, from -4.5 to +10.4 when the THP concentration was increased from 0 to 3%. In contrast, THP liposomes were much larger and their charge was not affected by THP. When compared with standard liposomes, ethosomes had a higher entrapment capacity and a greater ability to deliver entrapped fluorescent probe to the deeper layers of skin. The flux of THP through nude mouse skin from THP ethosomes (0.21 mg/cm2 h) was 87, 51 and 4.5 times higher than from liposomes. |
| |
CONCLUSION
|
| |
| Ethosomes are soft, malleable vesicles and potential carrier for transportation of drugs. Ethosomes are characterized by simplicity in their preparation, safety and efficacy and can be tailored for enhanced skin permeation of active drugs. Ethosomes have been found to be much more efficient at delivering drug to the skin, than either liposomes or hydroalcoholic solution. Ethosomes have been tested to encapsulate hydrophilic drugs, cationic drugs, proteins and peptides. Ethosomal carrier opens new challenges and opportunities for the development of novel improved therapies. |
| |
Conflict of Interest
|
| |
| None |
| |
Source of Support
|
| |
| Nil |
| |
Tables at a glance
|
 |
| Table 1 |
|
| |
Figures at a glance
|
 |
 |
| Figure 1 |
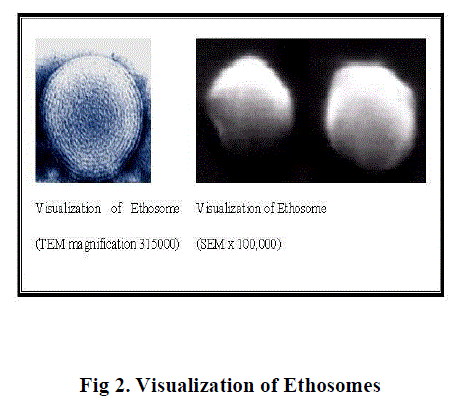
Figure 2 |
|
| |








