Keywords
Point-of-Care Testing (POCT); Rapid Plasma Separation Device (RPSD); Cortisol; D-dimer; Myoglobin; Thyroxine
Aim and Objective
Aim of this study was to evaluate Point-of-care i-CHROMA™ RPSD for estimations of cortisol, d-dimer, myoglobin and thyroxine.
Introduction
Blood sample preparation is an important step in the performance of blood testing. Plasma extraction from whole blood is essential when performing blood analysis. Centrifugation is the gold standard method for plasma extraction. Although the centrifugation technique is commonly used, it requires the sample to be sent to the lab [1]. In the last decade, intensive research to scale down experimental processes has seen unprecedented advances in filtration and point of care devices, such as the Sure-Sep device [2]. A selfdriven blood plasma separation microfluidic chip was shown to extract more than 0.1 μL of plasma from a single droplet of whole blood [3]. Recently, a novel pipette was described that can obtain 30 μL of plasma from 100 μL of whole blood within 5 minutes without any external equipment [4].
Many Point of Care Testing (POCT) devices are now available which means that testing can be done at the patient’s bedside or at the point of care for cortisol, thyroxine, d-dimer and myoglobin [5-7]. Many of these devices can perform these tests on whole blood samples but a large proportion of tests can only still be carried out on serum and plasma samples. The i-CHROMA™ is a novel fluorescence-based immunoassay that provides quantitative analysis of a range of tests in urine, serum, plasma or whole blood. The reaction is performed using disposable cartridges and the result is measured using the portable i-CHROMA™ Reader. It measures the following tests: Troponin, D-Dimer, CK-MB, Myoglobin, HsCRP, PSA, AFP, CEA, iFOB, HbA1C, Microalbumin, Cortisol, hCG, B-HCG, FSH LH, TSH, T3, T4, Prolactin, Testosterone, FSH, Progesterone, RF (IgM), ASO, CRP, HsCRP, Procalcitonin, Ferritin and Cystatin C. Recently, studies have been demonstrated the performance of the i-CHROMA™ with regards to its PSA, HCG, LH and FSH estimation [8,9]. For such tests to be performed there is still the challenge of separating plasma from the whole blood that is collected which adds another step of sample preparation.
The Rapid Plasma Separation Device (RPSD) uses a filtration method rather than centrifugation and therefore overcomes this problem and makes available plasma from a few micro liters to hundreds of micro liters from whole blood in a few minutes for Biochemical, Immunological as well as Molecular Biology assays. The RPSD device provides the recovery of liquid plasma within 3-5 minutes and is able to deliver more than rated amount of plasma at even up to 56% hematocrit. This method of extraction has several applications: For near patient testing where liquid plasma is required for qualitative and quantitative assays, for obtaining plasma for POC instruments and ambulances, for testing of patients for admission to acute care medical wards, for testing in remote areas where lab facilities are not available and in resource limited settings when centrifuge and electricity are not easily available [10]. The performance of the RPSD has been verified in immunological tests (HCV, HBsAg), biochemical tests (uric acid, urea and glucose), white blood cells and platelet count. The device has a unique design whereby whole blood is applied to a sample reservoir, the red blood cells (RBCs) are retained in the filter and plasma travels through a transport layer to an absorbent pad at the distal end. When the plasma-filled absorbent pad is pressed, liquid plasma is driven into the plasma collection well from where it can be collected (Figure 1) [10].
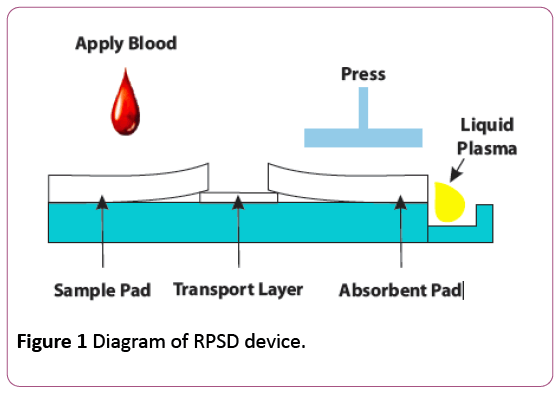
Figure 1: Diagram of RPSD device.
The objective of this study is to validate the integration of the RPSD-450 into the Cortisol, D-dimer, Myoglobin and thyroxine cartridges of the POC i-CHROMA™ immunoassay system by comparing (1) The recovery of plasma from whole blood using the RPSD-450 and (2) Comparison of estimations of Cortisol, D-dimer, myoglobin and thyroxine from RPSD plasma and Centrifuged plasma estimations using the i- CHROMA™ POCT Immunoassay system.
Materials and Methods
Specimens
Ten healthy volunteers were recruited, informed and consented to have their blood taken. The volunteers had been fasted for a minimum of 4 hours, drug free for a minimum of 7 days and had not consumed alcohol or smoked within 24 hours.
Blood was collected into lithium heparin tubes from each of them, haemoglobin and haematocrit estimated and then separated, 5 mL into two sets of tubes (A, B).
Tube’s A – whole blood was centrifuged and plasma collected (centrifuged plasma). Tube’s B – whole blood was applied to RPSD device (see RPDS method below) and plasma collected (RPSD plasma). The plasma samples were used for the respective analyte estimation using the i-CHROMA™ immunoassay system.
Rapid plasma separation device method
RPSD consists of a construction of filtration, transportation and absorption matrices embedded in a hinged plastic device. Whole blood was applied in a window provided in the device and plasma migrates to an absorption matrix through a transport matrix in 3-4 minutes. On pressing the hinged top, liquid plasma was expelled into a collection chamber where it was collected by a micropipette (see protocol below).
• 440 – 460 μL of whole blood was applied to the filter matrix embedded in a well as shown ensuring equal distribution in all the wells
• RBC was retained by the filter and the plasma moved across the transport matrix to absorption matrix and wait for plasma to collect and completely wet the distal end of the absorption matrix.
• The device was pressed to squeeze out free plasma into the reservoir.
• Free plasma was collected from the reservoir using a micropipette/capillary while the device remained in pressed condition.
Immunoassay estimations
The i-CHROMA™ system is a quantitative immunoassay system based on a fluorescence immunoassay technology. The i-CHROMA™ immunoassay systems uses a sandwich immunodetection method, such that the fluorescence labelled detector antibody or antigen binds to the target protein in sample. Signal intensity of fluorescence reflects the amount of the target protein captured and is micro processed from i- CHROMA™ reader to show the protein in the blood specimen.
i-CHROMA™ Cortisol, D-Dimer, Myoglobin and Thyroxine estimations
Cortisol
• 30 uL of plasma (centrifuged and RPSD) was taken from tubes A and B of each volunteer and transferred to a tube containing the detection buffer and mixed thoroughly.
• Seventy-five microliters (75 μL) of the sample mixture was pipetted and aliquoted into the sample well of the test cartridge.
• The test cartridge was left at room temperature for 10 minutes.
• The test cartridge was inserted into the i-CHROMA™ reader and the select button pressed, the i-CHROMA™ reader displayed the cortisol concentration in nmol/L and ug/dL on the LCD screen.
Thyroxine
• 10 μL of plasma (centrifuged and RPSD) was taken from tubes A and B of each volunteer and transferred to a tube containing Detection Buffer and mixed thoroughly.
• 75 μL was pipetted and aliquoted into the sample well of the test cartridge.
• The test cartridge was left at room temperature for 10 minutes.
• The test cartridge was inserted into the i-CHROMA™ reader and the select button pressed, the i-CHROMA™ reader displayed the myoglobin concentration in ng/mL on the LCD screen.
D-dimer
• 10 μL of plasma (centrifuged and RPSD) was taken from tubes A and B of each volunteer and transferred to a tube containing Detection Buffer and mixed thoroughly.
• 75 μL was pipetted and aliquoted into the sample well of the test cartridge.
• The test cartridge was left at room temperature for 12 minutes.
• The test cartridge was inserted into the i-CHROMA™ reader and the select button pressed, the i-CHROMA™ reader displayed the D-Dimer concentration in ng/mL on the LCD screen.
Myoglobin
• 10 μL of plasma (centrifuged and RPSD) was taken from tubes A and B of each volunteer and transferred to a tube containing Detection Buffer and mixed thoroughly.
• 75 μL was pipetted and aliquoted into the sample well of the test cartridge.
• The test cartridge was left at room temperature for 12 minutes.
• The test cartridge was inserted into the i-CHROMA™ reader and the select button pressed, the i-CHROMA™ reader displayed the myoglobin concentration in ng/mL on the LCD screen.
Results
From 1,760 microliters of whole blood used for 4 RPSD devices per volunteer using 440 μL per device, the average total amount of plasma recovered per RPSD ranged between 13.75 μL and 26.25 μL (Table 1). All the volunteers had normal haemoglobin and haematocrit values (Table 1) and the recovered plasma was generally lower in the volunteers with the higher heamatocrit (Figure 2).
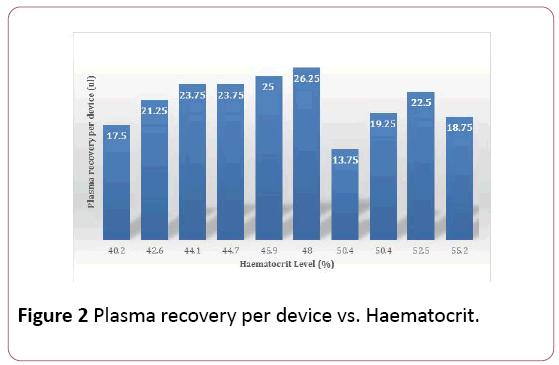
Figure 2: Plasma recovery per device vs. Haematocrit.
| Subject number |
Total amount of whole blood used (μL) |
Hbg (g/dL) |
Hct (%) |
Total amount of plasma collected from 4 RPSDs (μL) |
Average plasma harvested per RPSD |
| 1 |
1,760 |
16.8 |
50.4 |
55 |
13.75 |
| 2 |
1,760 |
13.4 |
40.2 |
70 |
17.5 |
| 3 |
1,760 |
18.4 |
55.2 |
75 |
18.75 |
| 4 |
1,760 |
16.8 |
50.4 |
77 |
19.25 |
| 5 |
1,760 |
15.3 |
45.9 |
100 |
25 |
| 6 |
1,760 |
14.2 |
42.6 |
85 |
21.25 |
| 7 |
1,760 |
14.9 |
44.7 |
95 |
23.75 |
| 8 |
1,760 |
14.7 |
44.1 |
95 |
23.75 |
| 9 |
1,760 |
17.5 |
52.5 |
90 |
22.5 |
| 10 |
1,760 |
16 |
48 |
105 |
26.25 |
Table 1: Hemoglobin, haematocrit, and average plasma harvested per RPSD.
Plasma cortisol (RPSD and centrifuged) estimations
The centrifuged plasma cortisol estimations for the volunteers (n=10) ranged between 157.3 nmol per litre and 694.94 nmol per litre. The RPSD plasma cortisol estimations for the volunteers (n=10) ranged between 194.11 nmol per litre and 692.47 nmol per litre. The RPSD plasma cortisol concentrations were comparatively higher in most of the volunteers. There was an average positive bias of 62.67 nmol/L, a bias difference of 17.4%. The RPSD plasma cortisol estimations correlated very well with their counterpart centrifuged plasma cortisol estimations (r2=0.97) (Figure 3). There was no statistical difference between the plasma cortisol concentrations (RPSD and their counterpart centrifuged plasma cortisol, shown using the Fisher T-Test (p=0.33) (Table 2).
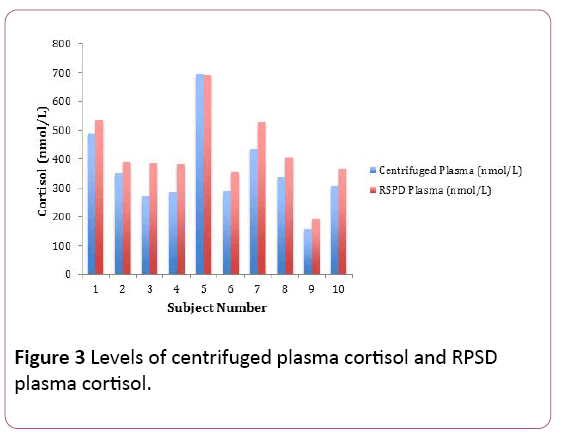
Figure 3: Levels of centrifuged plasma cortisol and RPSD plasma cortisol.
| Subject number |
Centrifuged Plasma Cortisol (nmol/L) |
RPSD Plasma Cortisol (nmol/L ) |
| 1 |
488.31 |
536.27 |
| 2 |
351.66 |
391.02 |
| 3 |
269.28 |
386.71 |
| 4 |
285.29 |
384.98 |
| 5 |
694.94 |
692.47 |
| 6 |
287.71 |
353.02 |
| 7 |
433.12 |
526.27 |
| 8 |
336.04 |
403.68 |
| 9 |
157.3 |
194.11 |
| 10 |
305.35 |
367.35 |
Table 2: Centrifuged plasma and RPSD plasma cortisol levels.
Plasma thyroxine (RPSD and centrifuged) estimations
The centrifuged plasma thyroxine estimations for the volunteers (n=10) ranged between 3.48 nanomoles per litre and 7.44 nanomoles per litre. The RPSD plasma thyroxine estimations for the volunteers (n=10) ranged between 1.94 nanomoles per litre and 6.14 nanomoles per litre. The RPSD plasma thyroxine estimations were comparatively lower in most of the volunteers.
There was a bias of -1.64 nmol/L and a bias difference of -31.7%. The results of the RPSD plasma thyroxine estimations correlated quite well with the centrifuged plasma thyroxine estimations (r2=0.67) (Figure 4). There was a statistical difference between the plasma thyroxine estimations (RPSD and centrifuged) shown using the Fisher T-Test (p=0.015) (Table 3).
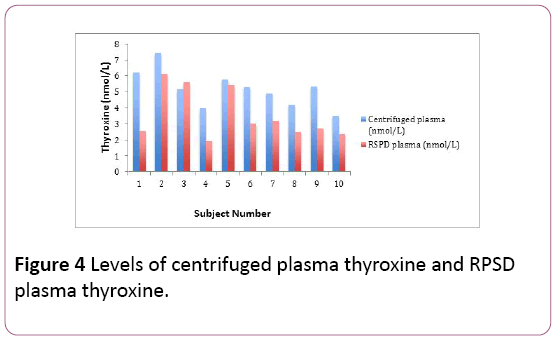
Figure 4: Levels of centrifuged plasma thyroxine and RPSD plasma thyroxine.
| Subject number |
Centrifuged plasma ng/mL |
RPSD plasma ng/mL |
| 1 |
6.2 |
2.54 |
| 2 |
7.44 |
6.14 |
| 3 |
5.17 |
5.59 |
| 4 |
3.98 |
1.94 |
| 5 |
5.76 |
5.41 |
| 6 |
5.3 |
3.01 |
| 7 |
4.9 |
3.18 |
| 8 |
4.19 |
2.48 |
| 9 |
5.33 |
2.71 |
| 10 |
3.48 |
2.34 |
Table 3: Centrifuged plasma and RPSD plasma thyroxine levels.
Plasma D-dimer (RPSD and centrifuged) estimations
The centrifuged plasma d-dimer concentrations for the volunteers (n=10) ranged between 442.74 nanograms per mL and 1379.85 nanograms per mL. The RPSD plasma d-dimer concentrations for the volunteers (n= 10) ranged between 148.16 nanograms per mL and 972.66 nanograms per mL. The RPSD plasma d-dimer concentrations were comparatively lower in most of the volunteers. There was an average bias of -382.4 ng/mL, and a bias difference of -48.15%. The results of the RPSD plasma D-Dimer estimations correlated well with the centrifuged plasma D-Dimer estimations (r2=0.88) (Figure 5). There was a statistical difference between the plasma d-dimer estimations (RPSD and centrifuged) shown using the Fisher TTest (p=0.006) (Table 4).
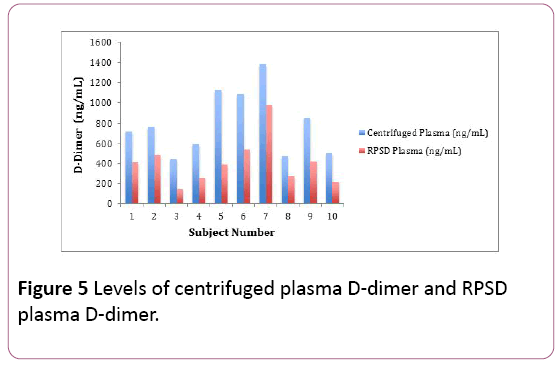
Figure 5: Levels of centrifuged plasma D-dimer and RPSD plasma D-dimer.
| Subject number |
Centrifuged plasma ng /mL |
RPSD plasma ng/mL |
| 1 |
718.42 |
414.55 |
| 2 |
764.04 |
487.68 |
| 3 |
442.74 |
148.16 |
| 4 |
592.19 |
254.73 |
| 5 |
1122.63 |
391.56 |
| 6 |
1084.74 |
542.23 |
| 7 |
1379.85 |
972.66 |
| 8 |
474.7 |
273.19 |
| 9 |
855.72 |
422.98 |
| 10 |
506.27 |
209.57 |
Table 4: Centrifuged plasma and RPSD plasma D-dimer levels.
Plasma myoglobin (RPSD and centrifuged) estimations
The RPSD plasma myoglobin estimations for the volunteers (n=7) ranged between 9.62 nanograms per mL and 41.92 nanograms per mL. The centrifuged plasma myoglobin estimations for the volunteers (n= 7) ranged between 15.84 nanograms per mL and 49.26 nanograms per mL. The RPSD plasma myoglobin estimations were comparatively lower in most of the volunteers. There was a bias of -5.66 ng/mL, and a bias difference of -22.57%. The results of the RPSD plasma myoglobin estimations correlated well with the centrifuged plasma myoglobin estimations (r2=0.98) (Figure 6). There was no statistical difference between the plasma myoglobin estimations (RPSD and centrifuged) shown using the Fisher TTest (p=0.43) (Table 5).
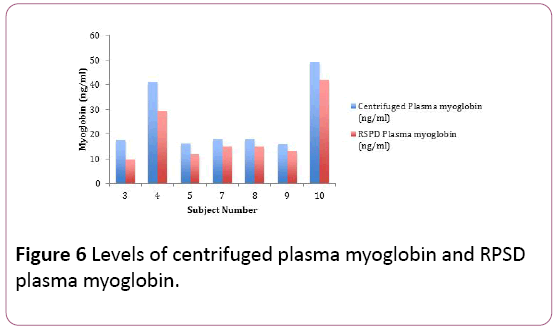
Figure 6: Levels of centrifuged plasma myoglobin and RPSD plasma myoglobin.
| Subject number |
Centrifuged plasma ng/mL |
RPSD plasma ng/mL |
| 3 |
17.61 |
9.62 |
| 4 |
40.99 |
29.41 |
| 5 |
16.11 |
11.82 |
| 7 |
17.99 |
15.1 |
| 8 |
17.75 |
14.94 |
| 9 |
15.84 |
13.11 |
| 10 |
49.26 |
41.92 |
Table 5: Whole blood, centrifuged plasma and RPSD plasma myoglobin levels.
Discussion
In this study, we were able to successfully recover between 13.75 – 26.25 μL per RPSD-450 device from 440 μL of whole blood applied. The thyroxine, d-dimer and myoglobin tests on the i-CHROMA™ POCT immunoassay system required 10 μL of plasma, while the cortisol test required 30 μL. From our assessment, we would suggest that one RPSD-450 is probably adequate enough to provide plasma for tests that require between 13 μL and 26 μL in patients with a haematocrit greater than 40% and up to 55%.
It has previously been shown that plasma derived from RPSD’s is quite comparable to plasma obtained by centrifugation for immunological, molecular biology and biochemistry applications [10]. In this study comparing immunoassay quantitative estimations for cortisol, thyroxine, myoglobin and d-dimer from plasma derived from RPSD’s compared to plasma obtained by centrifugation. Thyroxine, myoglobin and d-dimer concentrations obtained from plasma using the RPSD yielded significantly lower values compared to their plasma samples obtained by centrifugation. The most likely explanation for the lower concentration values seen with thyroxine, myoglobin and d-dimer could be that some portion of the analytes binds to the pad matrix of the RPSD, resulting in lower concentration estimates. These lower biases were in the order of -48.15% for d-dimer, -31.7% for thyroxine, -22.57% for myoglobin. These bias differences did not fit the desirable specification for allowable total errors: 28.4% for ddimer, -7.0% for thyroxine, -22.57% and 19.6% for myoglobin according to Westgard desirable specification for allowable total error [11]. There was a statistical difference between the d-dimer and thyroxine estimates from the RPSD derived plasma and the centrifuged derived plasma, but no statistical difference between the myoglobin estimates from the RPSD derived plasma and the centrifuged derived plasma. The estimated concentrations of thyroxine, myoglobin and d-dimer from plasma derived from RPSD’s compared to plasma obtained by centrifugation would not be considered acceptable.
The cortisol concentrations were higher in the plasma derived from the RPSD compared to estimations from the plasma samples obtained by centrifugation. An explanation for the higher estimates seen in the plasma derived from the RPSD could be because of the cortisol bound to the red blood cell surface. When the sample is applied to the RPSD, the pad matrix binding site in the RPSD competes with the binding site of the cortisol on red blood cells, causing the cortisol bound on the red blood cell to be displaced resulting in an increase in the plasma. This bias was +17.37% for the estimations and there was no statistical difference between the estimates from the RPSD obtained plasma and the centrifuged obtained plasma. According to Westgard the desirable specification for allowable total error is +/-22.8% [11]. The estimated concentrations of cortisol from plasma derived from RPSD’s compared to plasma obtained by centrifugation would be considered acceptable.
Conclusion
Although, the sample size of this study is small, it provides us some insight into the potential value of RPSD’s for POCT devices. We conclude that the cortisol estimations were comparable to cortisol estimations from plasma separated by centrifugation on the immunoassay point of care i-CHROMA™ Cortisol method and the bias acceptable. However, the ddimer, thyroxine and myoglobin estimations from the plasma derived from the RPSD450 were not comparable to the counterpart estimations from centrifuged plasma and unacceptable biases. The RPSD-450 could be potentially be used in near patient testing situations where liquid plasma is required for the quantitative analysis of cortisol.
20540
References
- Mukherjee S, Kang T, Chen Y, Kim S (2009) Plasma separation from blood: The ‘Lab on-a-chip’ Approach. Crit Rev Biomed Eng 37: 517-529.
- Garbien K, Tuttlebee J, Crowley M (1976) Evaluation of a serum/plasma Separator: Suitability of samples for radioimmunoassay and competitive protein binding techniques. Ann ClinBiochem 13: 449-451.
- Madadi H, Casals-Terre J, Mohammadi M (2015) Self-driven filter based blood plasma separator microfluidic chip for point of care testing Biofabrication 7: 025007.
- Im SB, Kim SC, Shim JS (2016) A smart pipette for equipment free separation and delivery of plasma for onsite whole blood analysis. Anal BioanalChem 408: 1391-139.
- KaushiK A, Vasudev A, Arya SK, Pasha SK, Bhansali S (2014) Recent advances in cortisol sensing technologies for point of care application. BiosensBioelectron 53: 499-512.
- Kim TK, Oh SW, Mok YJ, Choi EY (2014) Fluorescence immunoassay of human D-dimer in whole blood. J Clin Lab Anal 28: 294-300.
- Muller-Bardorff M, Sylven C, Rasmanis G, Jorgensen B, Collinson PO, et al.(2000) Evaluation of a point of care system for quantitative determination of troponin T and myoglobin. ClinChem Lab Med 38: 567-574.
- Bolodeoku J, Bains S, Chand V, Bacon R, Weir P, et al. (2017) A screening evaluation of the Point of Care Test (POCT) i-CHROMA Prostate Specific Antigen (PSA) assay method in the community. Point of Care: The Journal of Near Patient Testing and Technology 16 : 93–96.
- Bolodeoku J, Bains S, Pinkney S, Coker O, Fakokunde A (2017) Comparison of the point of care test (POCT), i-CHROMA human chorionic gonadotrophin (HCG), leutinizing hormone (LH) and follicle stimulating hormone (FSH) methods with the other laboratory methods in the Randox International Quality Assessment Scheme (RIQAS). ClinObstetGynacolReprod Med 3: 1-7.
- Westgard (2017) Desirable biological database specifications. Westgard QC Madison, Wisconsin, USA.












