Aseer Manilal1*, Gemechu Ameya1, Tigist Gezmu1, Behailu Merdekios1, Sabarathnam Balu2, Akbar Idhayadhulla3 and R. Surendra Kumar3
1College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
2Department of Comprehensive Dentistry, University of Texas Health Science Centre, San Antonio, Texas-78229, USA
3PG and Research Department of Chemistry, Nehru Memorial College, Puthanampatti -621007, Tamil nadu, South India
*Corresponding Author:
Aseer Manilal
Department of Medical Laboratory Sciences, College of Medicine and Health Sciences, Arba Minch University, Arba Minch, Ethiopia
Tel: 251-919904201
E-mail: aseermanilal@gmail.com
Received Date: Feb 01, 2016; Accepted Date: Feb 22, 2016; Published Date: Feb 25, 2016
Citation: Manilal A, Gezmu T, Merdekios B, et al. Evaluating the In Vitro Antagonism of Secondary Metabolites Fractionated from the Brown Algae, Sargassum swartzii Against Human Candida spp. Transl Biomed. 2016, 7:1. DOI: 10.21767/2172-0479.100051
Keywords
Algal fraction; Anticandidal activity; Secondary metabolites; Antimicrobials
Introduction
Fungi are the diverse group of ubiquitous eukaryotes which intercedes vital ecological processes. Nevertheless, many of them are primary or opportunistic pathogens capable of inflicting wide spectra of ailments in humans [1]. Amid the 1.5 million estimated species of fungi, around 317 species are known to smite diseases in human [2,3]. The fungal scourges have broad and variable clinical manifestations ranging from superficial skin and nails infections to disseminated life threatening infections [1]. In the developing countries, opportunistic fungi are oftentimes causing infections in immuno-compromised patients which therefore need to control promptly. Of the different infective species of fungi, Yeasts of the Candida genus inflict fatal systemic infections which increased substantially over the last decade. For instance, it is noted that the Candida spp. are the most common opportunistic pathogen in AIDS patients [4]. In addition, infections due to non-albicans species have also egressed over the past two decades, and a switch from C. albicans to species such as Candida glabrata, Candida parapsilosis, and Candida tropicalis has alarmingly increasing [5].
During the last decades, pharmaceutical industries have produced limited number of new therapeutic drugs for the management of Candida. However, the prolonged/inadequate usage of exciting antifungal drugs has precipitated the emergence of drug resistant Candida spp. and poses an extra concern [6]. With the increased incidence of Candidal infections coupled with the setback associated with the overuse and misuse of antibiotics demonstrates the insistent need of finding safe, novel and effective antifungal agents. Therefore, novel antifungal drugs with increased potency are required in lieu of conventional antibiotics for the management of fungal diseases.
In comparison to the chemicals and drugs used for synthetic treatments, allo-chemicals from marine origin are less associated with negative effects and have enormous therapeutic potentials to heal many infectious maladies in human [7]. Currently, marine organisms are an enormous reserve of novel drugs and drug leads for the pharmaceutical industry. Marine natural products have been discovered from a wide array of organisms including sponges, algae, bryozoans, mollusks, cnidarians, tunicates, echinoderms, sea worms and microorganisms. It is a confirmed fact that, the marine algae are prime candidate in producing antimicrobial metabolites to thwart the invaders in their natural habitat. Pluralities of algal species have been reported to possess multitude of bioactivities and thence, potential for elaborating antimicrobial agents. For instance, literature addressed that species of genus Sargassum presented diverse activities such as anti-Herpetic [8], antiretroviral [9], antifungal [10], antibacterial [11], and anticancer [12].
Retrospective examination evidenced that antifungal actions of marine algae were exposited as earlier as 1915s [13]. Various marine algae from the Southwest littoral of India has been recently corroborated for exerting diverse bioactivities such as antibacterial [14], antifungal [15], antiviral [16], anticoagulant [17] and cytotoxicity [18]. In so far, there is no precedence of research being conducted to inspect the antagonistic potential of brown algae, Sargassum swartzii against human Candida. In our preliminary experiments, the crude methanolic extract of S. swartzii evinced pronounced antibacterial activity against clinical and biofilm forming bacteria. In this regard, the brown algae S. swartzii was preferred to explore its anticandidal potency and GC-MS analysis to delineate its bioactive principles.
Materials and Methods
Collection of algal specimens
Live and healthy thalli of marine algae, S. swartzii were handpicked at ebb tide from the rugged intertidal zone of Kollam coast, South India (08°54’ N and 76°38’ E). Garnered specimens were then successively rinsed with seawater to remove dirt and transferred to laboratory in plastic bags containing seawater to prevent evaporation. The rinsed thalli were air dried under a stream of air flow for one week at room temperature to prevent photolysis and thermal degradation of metabolites. Dried fronds of algae were powdered in a coffee grinder, packed in polyethylene bags and stored in moisture free place until extraction
Extraction of algae
Algal bioactives were extracted from dried algal powder according to the parameters previously optimized [14]. Briefly, a definite quantity (200 g) of dried algal powder was submerged in conical flasks (2000 mL) containing 1000 mL of methanol (MeOH) and placed at 35°C on a shaker at 120 rpm for two weeks to permit full extraction of the bioactive components. After two weeks, algal material was filtered using Whatman filter paper No 1. The filter residue was collected in a round-bottom flask and the solvent was concentrated in a rotary vacuum evaporator at 45°C for the elimination of MeOH. The resultant gummy dark coloured extract was collected in airtight plastic vials and stored in the refrigerator for further studies.
Fractionation of Sargassum swartzii
A known morsel of dried extract of the algae (crude solid residue collected after vacuum evaporation) was adsorbed to silica gel and applied in a column developed with petroleum ether and eluted step-wise with petroleum ether and ethyl acetate (9:1 to 1:9 and 100% ethyl acetate) followed by ethyl acetate and methanol (9:1 to 1:9 and 100% methanol) [15]. Column elute was collected in 10 mL screw cap bottles. Preliminary experiments confirmed that the fraction eluted using ethyl acetate: methanol (4:6) retained antibacterial activity (data not shown). Other fractions that displayed meagre activity were not more considered in the present study. Hence, the same fraction (4:6) was used to investigate anticandidal activity and GC-MS analysis.
Gas chromatographic and mass spectroscopic (GC-MS) analysis
The active fraction was chemically analysed through GC-MS method as described elsewhere [14].
Antifungal assay
To determine anticandidal activity, the pre-selected algal fraction was evaluated against a battery of clinically relevant five species of Candida those previously used and continuously maintained in our laboratory [15]. The anticandidal assay was performed as described elsewhere [15]. The Sabouraud dextrose agar (Himedia) was used for bioactivity screening and routine propagation of human fungus respectively. Cell suspensions containing 107 CFU/ml cells for yeasts, were prepared and aseptically besmeared onto the surface of the agar plates of Sabouraud dextrose medium using sterile swab sticks. Thereafter, wells with five millimeter of diameter were prepared using a sterile cork borer. The resultant wells were carefully filled with 100 μl (15 mg/ml) of algal fraction. The well with binary solvents used for fractionation was considered as negative control. The assay was performed in triplicates of individual Petri dishes. The clear zones of inhibition formed around wells after 48 h at 30°C were considered to be an indicative of anticandidal activity. The inhibitory activity was recorded by calculating the area of clear zone and anti-biogram was statistically analyzed.
Statistical analysis
The results are expressed as means ± SE of three experiments. Mean values were assessed using One-way analysis of variance using SPSS for Windows version 20.0 (Statistical Package for Social Services, Chicago, IL, USA).
Results
Our preliminary study posited that, the methanolic extract of S. swartzii was excellent for subduing the growth of clinical and biofilm forming bacteria in vitro (data not shown). And there is an extreme paucity of studies pertains to the anticandidal efficacy of S. swartzii from the Indian littoral. Thence, in the present study, anticandidal activity of S. swartzii was explored against the panel of five species of Candida. The overall results emphasize the potentiality of using S. swartzii for the development of chemotherapeutic agents against Candida sp. Figure 1 illustrates the inhibitory spectra produced by the S. swartzii against Candida spp. It is evident that the S. swartzii exerted broad spectra of activity against all the tested species of Candida in varying degrees. The active algal fraction produced mean zones of inhibition ranged between 102.17 ± 10.03 mm2 to 117.51 ± 22.28 mm2 against the Candida sp. The anticandidal action was very high against C. albicans to the extent of 117.51 ± 22.28 mm2. The nearly active range was displayed against C. tropicalis (110.37 ± 17.7) and C. glabrata (107.03 ± 23.45). The activity range of C. krusie and C. parapsilosis accounted for 100.86 ± 20.5 and 102.17 ± 10.03 mm2 respectively. The overall results exposited that potent anticandidal constituent can be isolated from S. swartzii.
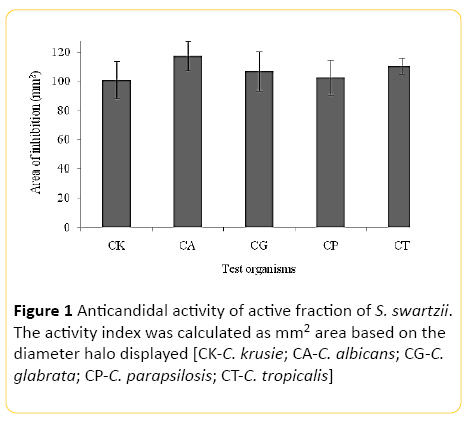
Figure 1: Anticandidal activity of active fraction of S. swartzii. The activity index was calculated as mm2 area based on the diameter halo displayed [CK-C. krusie; CA-C. albicans; CG-C. glabrata; CP-C. parapsilosis; CT-C. tropicalis]
GC-MS analysis of active fraction of S. swartzii
The active fraction was subjected to GC-MS analysis to explicate its bioactive chemical constituents. The spectral data has brought a single prominent peak to fore with the retention time and molecular weight of 25.73 and 390 respectively (Table 1). MS data has perfectly matched a compound of molecular formula C24H38O4 that is analogous to 1,2- Benzenedicarboxylic acid, diisooctyl ester in the NIST library.
| No |
RT |
Name of the compound |
MF |
MW |
PA (%) |
| 1 |
14.44 |
3, 7, 11, 15-Tetramethyl-2-hexadecen-1-ol |
C20H40O |
296 |
6.10 |
| 2 |
16.24 |
n-Hexadecanoic acid |
C16H32O4 |
256 |
16.36 |
| 3 |
25.73 |
1, 2-Benzenedicarboxylic acid, diisooctyl ester |
C24H38O4 |
390 |
77.54 |
| |
|
|
|
|
100 % |
RT-Retention time; MF-Molecular formula; MW-Molecular Weight; PA-Peak Area
Table 1 Components identified in the active fraction of S. swartzii by GC-MS study
Discussion
Globally, diseases caused by the species of genus Candida are leading health problems with high morbidity in immunocompetent and immuno-compromised patients [19]. The application of chemotherapeutics for the management of clinically relevant Candida spp. is currently restricted by the development of drug resistance. In this viewpoint, an antifungal agent with broad efficacy and minimal side effect is needed to mitigate the plights of vast masses of immunocompetent and immuno-compromised patients.
Bioactive molecules of marine algal origin have high potentiality to subjugate the growth of many infectious organisms. In fact, several in vitro studies are demonstrated the anticandidal activity of many marine algae [15,20-22]. Albeit, diverse species of Sargassum from the Indian coast has been recognized as a potential source of antibacterial agents, the anticandidal activity has seldom been reported [23]. Therefore, in the present study, secondary metabolites fractionated from S. swartzii were quantitatively examined anticandidal efficacy. The algal fraction exerted wider spectrum of activity, since it impeded the growth of all the evaluated Yeast pathogens in variable degrees. Anticandidal activity was found to be positively skewed toward C. albicans as compared to other Yeast spp. This type of variation in the efficacy was analogous to that observed for red algae, A. taxiformis against human Candida spp. [15]. In accordance with the present study, the crude extract of other sp. of Sargassum exhibited antifungal activity against C. albicans [24]. The demonstration of anticandidal efficiency is an indication that the S. swartzii is a potential source for bioactive-compounds with broad spectra of activity. The secondary metabolites extracted from the algae can incapacitate the growth of Yeast by mechanisms that are unlike those of antifungal agents currently available. Thence, it is posited to have significant clinical value in the management of resistant yeast strains. Howbeit, further detailed studies are necessary to verify the in vivo efficacy and mechanism of action.
The antimicrobial compounds responsible for the anticandidal efficacy are not elucidated in this study. Howbeit, GC-MS analysis of active fraction evinced the presence of three compounds such as, 1,2-Benzenedicarboxylic acid, diisooctyl ester (390 g/mol), n-Hexadecanoic acid (256 g/mol) and 3, 7, 11, 15-Tetramethyl-2-hexadecen-1-ol (296 g/mol) Thence, it is envisaged that the growth inhibition of Candida spp. displayed by the active algal fraction could be due to the presence of major principle 1,2-Benzenedicarboxylic acid, diisooctyl ester, or could be related with synergistic activity of all components, since antimicrobial activities are pertained to the presence of secondary metabolites. The bioactive phytochemicals of diverse species of genus Sargassum were well reviewed by Liu et al. [25]. The same author noted the presence of 1,2-Benzenedicarboxylic acid; Dioctyl ester in S. wightii. Similarly, the GC-MS results are in line with recent studies that annotated the similar bioactive chemical constituents in other plant specimens [26-29].
Conclusion
Hitherto, there is no report on the anticandidal activity of S. swartzii against the five species of clinically relevant Candida. Hence, this report is the first to explore the anticandidal activity of S. swartzii from the South Indian littoral. The present findings disclose that the brown algae, S. swartzii is potential to oppress the growth of all tested fungal pathogens in vitro. The prevalence of anticandidal activity of S. swartzii reflects the credible evidence that algae hold effective anticandidal chemical defences. In this context, more studies pertaining to mode of action of algal bioactives and interaction with pathogenic fungi may bring forth new drug leads for the control of fungal pathogens.
8795
References
- Köhler JR, Casadevall A, Perfect J (2014) The spectrum of fungi that infects humans. Cold Spring HarbPerspect Med3: 5.
- Garcia-Solache MA, Casadevall A (2010) Global warming will bring new fungal diseases for mammals. mBio 1: 1.
- Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res105:1422-32.
- Sanchez-Vargas LO, Ortiz-Lopez NG, Villar M, Moragues MD, Aguirre JM, et al.(2005)Point prevalence, microbiology and antifungal susceptibility patterns of oral Candida isolates colonizing or infecting Mexican HIV/AIDS patients and healthy persons. Rev IberoamMicol22: 83-92.
- van Asbeck EC, Clemons KV, Stevens DA (2009)Candidaparapsilosis: a review of its epidemiology, pathogenesis, clinical aspects, typing and antimicrobial susceptibility. Crit Rev Microbiol 35: 283-309.
- Morace G,Perdoni F, Borghi E (2014)Antifungal drug resistance in Candida species. J Glob Antimicrob Resist 2: 254-259.
- Manilal A, Sujith S, Selvin J, Shakir C, Gandhimathi R, et al. (2010) Antimicrobial potential of marine organisms collected from southwest coast of India against multiresistant human and shrimp pathogens. Sci Mar 74: 287-296.
- Zhu W, Chiu LC, Ooi VE, Chan PK, Ang PO Jr (2006) Antiviral property and mechanisms of a sulphated polysaccharide from the brown alga Sargassum patens against Herpes simplex virus type 1. Phytomed13:695-701.
- Paskaleva EE,Lin X, Duus K, McSharry JJ, Veille JL, et al.(2008) Sargassum fusiforme fraction is a potent and specific inhibitor of HIV-1 fusion and reverse transcriptase.Virol J5:8.
- Oranday MA, Verde MJ, Martinez-Lozano SJ, Waksman NH (2004) Active fractions from four species of marine algae. Phyton Int J Exp Bot 165-170.
- Chiao-Wei C, Siew-Ling H, Ching-Lee W (2011) Antibacterial activity of Sargassumpolycystum C.Agardh and Padina australis Hauck (Phaeophyceae).Afr J Biotechnol 10: 14125-14131.
- Yamaguchi M, Matsumoto T (2005) Marine algae Sargassum horneri bioactive factor suppresses proliferation and stimulates apoptotic cell death in human breast cancer MDA-MB-231 cells in vitro. Integr Mol Med.
- Pratt R, Mautner R, Gardener GM, Sha Y,Dufrenoy J (1951) Report on antibiotic activity of seaweed extracts. J Amer Pharm Asst Sci 40:579-579.
- Manilal A, Sujith S, Selvin J, Kiran GS (2010) Antibacterial activity of Falkenbergiahillebrandii (Born) from the Indian coast against human pathogens. Phyton Int JExp Bot 78: 161-166.
- Manilal A,Sujith A, Kiran GS,Selvin J, Shakir C, et al. (2009) Antimicrobial potential and seasonality of red algae collected from southwest coast of India tested against shrimp, human and phytopathogens. Ann Microbiol 59: 207-219.
- Manilal A, Sujith A, Kiran GS,Selvin J,Shakir C (2009)In vivo Antiviral Activity of Polysaccharide from the Indian Green Alga, Acrosiphoniaorientalis (J. Agardh): Potential Implication in Shrimp Disease Management. World J FisMar Sci 1: 278-282.
- Manilal A,Sujith S,Selvin J, Panikkar MVN, George S (2012) Anticoagulant potential of polysaccharide isolated from Indian red alga,Asparagopsistaxiformis (Delile) Trevisan. Thalassas Inter J Mar Sci 28: 9-15.
- Manilal A,Sujith A, Kiran GS,Selvin J, Shakir C (2009) Cytotoxic potentials of Laurencia brandeniicollected from the Indian Coast. Global J Pharmacol 3: 90-94.
- Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, et al. (2009) Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503-35.
- Ismail A, BelHadj Salah K, Ahmed M, Mastouri M, Bouraoui M, et al. (2014)Antibacterial and antifungal activities of brown alga Zonariatournefortii (J.V. Lamouroux). Allelopath J34: 143-154.
- Indira K, Balakrishnan S, Srinivasan M, Bragadeeswaran S,Balasubramanian T (2013) Evaluation of in vitro antimicrobial property of seaweed (Halimeda tuna) from Tuticorin coast, Tamil Nadu, Southeast coast of India. Afr J Biotechnol 12: 284-289.
- Elnabris1 KJ, Elmanama AA, Chihadeh WN (2013) Antibacterial activity of four marine seaweeds collected from the coast of Gaza Strip, Palestine. Mesopot J Mar Sci 28: 81-92.
- Prabhakar K, Sathish Kumar L, Rajendran S, Chandrasekaran M, Bhaskar K, et al. (2008) Antifungal Activity of Plant Extracts against Candida Species from Oral Lesions Indian. J Pharm Sci 70: 801-803.
- El-Sheekh MM, Gharieb MM, El-Sabbagh SM, Hamza WT (2014) Antimicrobial Efficacy of Some Marine Macroalgae of Red Sea. Int J Microbiol Immunol Res 3: 021-028.
- Liu L, Heinrich M, Myers SP,Dworjanyn SA (2012) Towards a better understanding of medicinal uses of the brown seaweed genus Sargassum in traditional Chinese medicine: a phytochemical and pharmacological review.JEthnopharmacol 142: 591-619.
- Krishnamoorthy K,Subramaniam P (2014) Phytochemical Profiling of Leaf, Stem, and Tuber Parts of Solenaamplexicaulis (Lam.) Gandhi Using GC-MS. Int Sch Res Notices.
- Padmalochana K, DhanaRajan MS, Lalitha R, Sivasankari H (2013) Evaluation of the Antioxidant and Hepatoprotective Activity of CryptolepisBuchanani.J App Pharm Sci 3: 99-104.
- Al-Shammaria LA, Hassanab WHB, Al-Youssefa HM (2012) Chemical composition and antimicrobial activity of the essential oil and lipid content of Carduuspycnocephalus L. growing in Saudi Arabia. J Chem Pharm Res4:1281-1287.
- Rajamurugan R, Selvaganabathy N,Kumaravel S, Ramamurthy CH,Sujatha V, et al. (2011) Identification, quantification of bioactive constituents, evaluation of antioxidant and in vivo acute toxicity property from the methanol extract of Vernoniacinerea leaf extract. Pharm Biol 49: 1311-1320.






